All published articles of this journal are available on ScienceDirect.
Effectiveness of Intermittent Feeding Combined with Right Lateral Position on Gastric Residual Volume in Critically Ill Patients: A Randomized Controlled Study
Abstract
Introduction/objective
Gastric residual volume is considered a monitoring parameter for impaired gastric emptying and enteral nutrition tolerance in ICU patients. Changing the body position to the right lateral position is regarded as an alternative approach that may accelerate gastric emptying. This study aimed to evaluate the effectiveness of enteral nutrition using the intermittent feeding method combined with the right lateral position on gastric residual volume in ICU patients.
Methods
This study was an open-label randomized controlled trial involving 52 ICU patients who were allocated to the control group (n=26) and the intervention group (n=26). The intervention group received enteral nutrition using intermittent feeding combined with the right lateral position, while the control group received standard care. The study was conducted from March 16 to June 14, 2024. Monitoring of gastric residual volume was conducted six times daily using an observation sheet, each time before the administration of enteral nutrition, for three consecutive days. The data were analyzed using statistical tests including frequency distribution, the Mann-Whitney U test, Spearman’s rank correlation, and linear regression.
Results
The results showed no difference between enteral nutrition using the intermittent feeding method combined with the right lateral position and standard treatment in terms of gastric residual volume (Mean [SD]: 306.25 [302.94] mL vs 315.38 [342.16] mL; p = 0.927). Additionally, this study found that the administration of catecholamine drugs and blood potassium levels were significantly associated with gastric residual volume in the intervention group (p = 0.035 and p = 0.022, respectively).
Discussion
Performing the right lateral position in ICU patients during intermittent feeding may serve as a strategy to reduce gastric residual volume, as indicated by the lower volume observed compared with the semi-recumbent position.
Conclusion
No statistically significant differences were found between the two groups regarding gastric residual volume. The use of catecholamines and potassium levels may be important factors to consider in determining enteral nutrition tolerance and the rate of gastric emptying. However, further studies with larger sample sizes are recommended to strengthen these findings.
1. INTRODUCTION
Nutritional support plays an important role for critically ill patients mainly because they are at high risk of malnutrition [1-3]. The prevalence of malnutrition in critically ill patients in a Brazilian hospital was estimated at 54% [4]. Another previous study found that among patients who received enteral nutrition more than 48 hours after admission to the Intensive Care Unit (ICU), 28.6% developed malnutrition, and 84% of them failed to achieve a minimum of 80% of their protein and energy targets [5]. This circumstance highlights the high demand for enteral nutrition via nasogastric tube (NGT) insertion to meet nutritional needs, especially for mechanically ventilated ICU patients.
In the context of enteral nutrition, intermittent feeding has been recognized as the most appropriate method due to its ability to maintain normal digestive physiology [6, 7]. It is important to note that 80% of ICU patients with NGTs experience high gastric residual volume (GRV), which contributes to nutritional intolerance [3, 8, 9]. GRV is considered a parameter for monitoring impaired gastric emptying and enteral nutrition tolerance in ICU patients [10]. However, the incidence of increased GRV during enteral nutrition administration in critically ill patients remains unresolved [11].
Adjusting the patient’s body position by maintaining the head of the bed at a 45-degree angle or using the semi-recumbent position is recommended to reduce the risk of gastric aspiration [12]. Additionally, the right lateral position is considered to accelerate gastric emptying [13]. Only a few studies have evaluated the effect of right lateral positioning during enteral nutrition on reducing GRV. One previous study investigated right lateral positioning compared with the semi-recumbent position alone [14]. No existing studies have specifically examined the effect of right lateral positioning during enteral nutrition using the intermittent feeding method in adult critically ill patients. At present, such interventions have only been studied in neonatal populations [15]. Hence, there is a significant gap to be addressed. This study may offer novelty as the first to evaluate the combined intervention of intermittent enteral feeding and right lateral positioning on GRV in adult ICU patients.
2. MATERIALS AND METHODS
2.1. Study Design
This study was an open-label randomized controlled trial with a posttest-only control design. Due to the nature of the intervention, which involved intermittent feeding and patient positioning, blinding of participants and researchers was not feasible. The posttest-only design was selected because GRV, as the dependent variable, cannot be measured or meaningfully interpreted before the intervention. Baseline measurement of GRV was neither possible nor ethical without prior exposure to feeding or intervention. Therefore, a pretest could not be conducted.
This manuscript was prepared following the CONSORT (Consolidated Standards of Reporting Trials) 2010 guidelines to ensure transparent and comprehensive reporting of RCTs.
2.2. Participants and Sampling Methods
The population in this study consisted of all critically ill patients who were mechanically ventilated in the ICU of Persahabatan Hospital, Indonesia. Samples were obtained using consecutive sampling. The inclusion criteria were: 1) age > 18 years; 2) newly admitted ICU patients who were mechanically ventilated; 3) planned to receive enteral nutrition via a nasogastric tube (NGT) or orogastric tube (OGT); and 4) receiving enteral nutrition 24 hours after mechanical ventilation. The exclusion criteria were: 1) pregnancy; 2) receiving enteral nutrition via nasojejunal, jejunostomy, or gastrostomy tubes; 3) contraindications for lateral or right oblique positioning; and 4) BMI < 18.5 kg/m2.
The sample size was calculated to achieve 80% power with a two-sided α = 0.05. Two steps of calculation were performed to justify the final sample size. First, the Lwanga & Lemeshow single-proportion formula was applied using a conservative proportion (p = 0.5) and acceptable margin of error (d = 0.20):
This resulted in 24 subjects per group for estimating a proportion with ±20% precision under maximum variance (p = 0.5). Second, because the hypothesis compared two independent groups on a continuous outcome (gastric residual volume), the two-sample t-test approach based on standardized effect size (Cohen’s d) was considered. A sample of n = 24 per group was deemed sufficient to detect a large effect (Cohen’s d ≈ 0.8) with 80% power and α = 0.05. To anticipate potential dropouts, 10% attrition was added, resulting in a final target of 26 participants per group (52 total).
Respondents were assigned to the experimental group (n = 26) and the control group (n = 26) through simple randomization to minimize bias. Simple randomization was performed by the primary researcher by randomly drawing a paper numbered 1 to 52 from a plastic cup. If the number drawn was even, the respondent was allocated to the control group; if odd, the respondent was allocated to the experimental group. To mitigate selection bias, allocation concealment was maintained by ensuring that group assignment (even or odd) was not disclosed to the research assistant or ICU staff until after the number was drawn.
The researcher provided an explanation of the study to the patient’s family member or guardian and requested their consent for participation. Those who agreed signed the informed consent form.
At the beginning of the study, 64 respondents were recruited (32 in each group) to anticipate potential dropouts. During the study, 12 respondents dropped out (5 from the intervention group and 7 from the control group) due to extubation, transfer to a regular care ward, hemodynamic instability, gastric bleeding, gastric residual volume > 500 mL, or the need to fast for surgical or diagnostic procedures. After these dropouts, 52 respondents (26 in each group) completed the study protocol. No replacements were added after dropouts; initial over-recruitment ensured the final sample met the target size.
The flow of respondent selection is shown in Fig. (1).
2.3. Instrument
In this study, the data collection tools or instruments used were a respondent characteristic form and an enteral nutrition observation form. The respondent characteristic sheet contains information on respondent data regarding age, gender, Acute Physiology and Chronic Health Evaluation (APACHE) II score, and blood potassium levels. Data on age, gender, APACHE II score, and blood potassium levels were taken using initial ICU admission data obtained from electronic medical records.
The observation sheets contain monitoring of enteral nutrition provision documented at the beginning and end of administration, type of nutritional formula, and volume of enteral nutrition given at predetermined hours. This instrument also states whether the patient received catecholamine drugs (dobutamine, dopamine, norepinephrine, epinephrine) at each intervention in both the experimental and control groups.
The researcher conducted the Interclass Correlation Coefficients (ICC) test calculation on the respondent characteristics instrument using SPSS to align perceptions with the research assistant regarding filling out the instrument and monitoring the research procedure, and obtained a result of 0.889. This ICC score indicates excellent inter-rater reliability, which means the agreement between the researcher and assistant is considerably high.
2.4. Intervention
Respondents were divided into two groups: the experimental group (n = 26) and the control group (n = 26). Researchers and research assistants ensured that hemodynamic conditions were stable before administering enteral nutrition. Criteria for stable hemodynamics included a mean arterial pressure (MAP) > 65 mmHg, no abdominal distension, diarrhea, or gastric bleeding. Additionally, the patency of the NGT, the use of catecholamine drugs (dobutamine, dopamine, norepinephrine, epinephrine), and the presence of bowel sounds were checked. Both intervention and control groups received enteral nutrition 24 hours after ICU admission. The type of daily enteral nutrition formula followed the regimen recommended by a nutrition specialist.
Respondents were positioned in a semi-recumbent/semi-Fowler position 15 minutes before the start of nutrition administration. Gastric residual volume (GRV) measurements were performed in the same manner for both groups. GRV was measured by aspirating gastric contents using a 50 mL syringe (catheter tip). If the GRV was <500 mL, enteral nutrition was administered unless the patient experienced nausea, vomiting, bloating, abdominal distension, or severe abdominal pain, in which case nutrition was temporarily withheld. If the GRV was >500 mL, enteral nutrition was postponed, and the GRV was re-evaluated after 2 hours. Nutrition was resumed when GRV was <500 mL; if GRV remained >500 mL, nutrition continued to be postponed, and consultation with a nutrition specialist or dietitian was conducted.
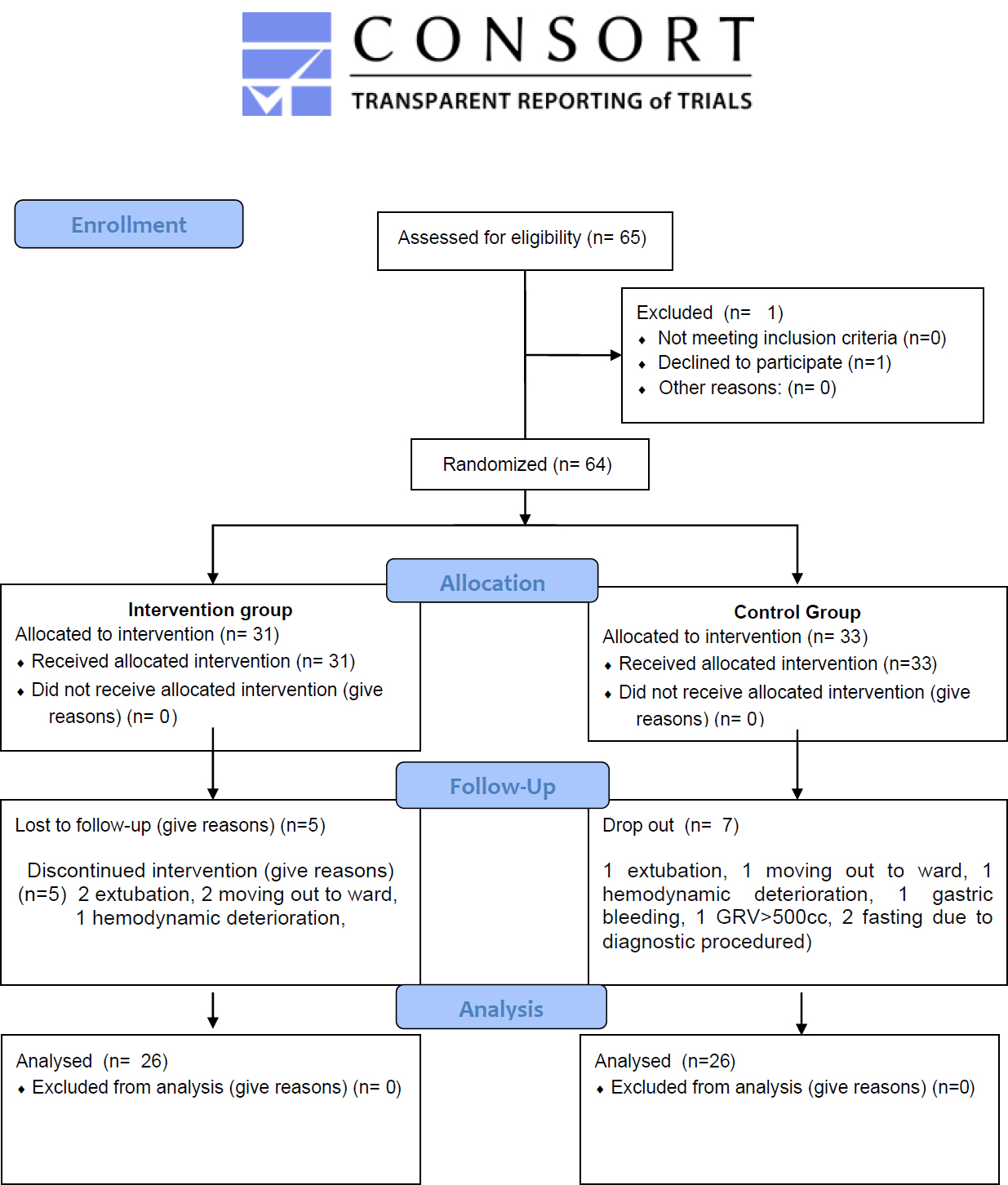
Participant’s flow.
2.5. Experimental Group
In the experimental group, after measuring gastric residual volume and confirming that enteral nutrition could be administered, patients were positioned in the right lateral position with a 45-degree head elevation. Enteral nutrition was delivered using the intermittent feeding method over 60 minutes via gravity drip/infusion (feeding burette) suspended on an infusion pole approximately 30 cm above the NGT insertion site. The feeding burette was a 100 mL nutrient container made of safe, BPA-free material, equipped with measurement lines, a hose, and a roll clamp at the bottom. The administration rate was controlled by adjusting the roll clamp, and the volume was given according to the patient’s nutritional needs six times per day. The volume of enteral nutrition administered started at 50 mL per feeding on the first day, 100 mL on the second day, and 150 mL on the third day of the study, at 07:00, 11:00, 15:00, 19:00, and 03:00.
The right lateral position was maintained for 60 minutes after each intermittent feeding, after which the patient was returned to the semi-recumbent position. To minimize observer variability, all procedures were performed by trained ICU nurses under the supervision of the research team according to a standardized protocol. Patient positioning, feeding setup, and drip regulation were continuously monitored to ensure uniformity across participants.
2.6. Control Group
In the control group, respondents received standard care, which consisted of maintaining a semi-recumbent position with a 45-degree head elevation both before measuring gastric residual volume and during enteral nutrition. Enteral nutrition was administered using the bolus feeding method via a catheter tip with a short gravity drip (3–5 minutes). The volume and timing of enteral nutrition administration were the same as in the experimental group.
2.7. Data Collection
The study was conducted in the ICU ward of Persahabatan Hospital, East Jakarta, from March 16 to June 14, 2024. After obtaining ethical approval from the Health Research Ethics Committee (HREC) of Persahabatan Hospital, the researcher recruited three adult ICU nurses as research assistants. Inclusion criteria for research assistants were: holding a Bachelor's degree in Nursing, at least three years of adult ICU experience, working in shifts, and having a schedule aligned with the study period.
To ensure consistency, the researcher provided training and guidance to the research assistants on general research procedures, administering enteral nutrition using the intermittent feeding method, positioning patients in the right lateral position, and documenting respondent characteristics and observation data. The researcher and assistants identified eligible patients for inclusion in the study.
Respondents were then assigned to the experimental or control group using simple randomization. After obtaining consent from the patient’s family member or guardian, the research team collected baseline data including age, gender, APACHE II score, type of enteral nutrition formula, and blood potassium levels from electronic medical records. Additionally, the use of catecholamine drugs (dobutamine, dopamine, norepinephrine, epinephrine), the volume of enteral nutrition administered, and gastric residual volume measurements were recorded on the observation sheet.
Interventions in the experimental group, consisting of intermittent feeding combined with the right lateral position, were conducted six times per day (every four hours) for three consecutive days, as long as enteral nutrition could be provided in the ICU. Standard interventions were provided to the control group according to protocol.
2.8. Data Analysis
Univariate analysis for numerical data used measures of central tendency, including mean, median, and standard deviation, while categorical data were analyzed using proportions or percentages. The normality of numerical data was assessed using the Kolmogorov-Smirnov test, as the number of respondents was greater than 50. Bivariate analysis for numerical data employed Spearman’s rank correlation, while categorical data were analyzed using the Mann-Whitney U test; for categorical data with more than two groups, the Kruskal-Wallis test was used. Statistical decisions were based on the significance value (p-value), with p ≤ 0.05 considered statistically significant. Linear regression analysis was employed to determine the effect of independent variables on gastric residual volume. All analyses were performed using IBM SPSS Statistics software version 25.
2.9. Ethical Considerations
This study received ethical approval from the Health Research Ethics Committee (HREC) of Persahabatan Hospital (letter number 0031/KEPK-RSUPP/02/2024) and was conducted in accordance with the Declaration of Helsinki. However, the study was not registered in a public clinical trial registry, as it was a single-site academic study and registration was not required by the ethics committee at the time of approval.
3. RESULTS
3.1. Characteristics of Respondents
Table 1 shows that the average age of respondents in the experimental group was 58.08 years (SD = 14.05) and in the control group was 58.81 years (SD = 10.97). The youngest respondent in the experimental group was 26 years and the oldest was 81 years, while in the control group, ages ranged from 31 to 74 years. Male and female respondents were evenly distributed, with a proportion of 50% each. In the experimental group, the average APACHE II score was 20.27 (SD = 7.31), compared with 17.92 (SD = 8.80) in the control group. The majority of respondents received catecholamine drugs (57.7%) and other types of enteral nutrition formulas (53.8%). Analysis of blood potassium levels showed an average of 4.47 mEq/L (SD = 0.78) in the experimental group and 4.17 mEq/L (SD = 0.92) in the control group.
3.2. Effectiveness of Intermittent Feeding and Right Lateral Position on GRV
Figure 1 illustrates the mean GRV across study days, showing a slightly lower trend in the experimental group, although confidence intervals overlapped widely. Table 2 provides a description of respondents based on gastric residual volume measured from the first to the third day of the study. The average cumulative GRV was similar between groups: 306.35 ± 302.94 mL in the experimental group and 315.38 ± 342.16 mL in the control group.
The Mann-Whitney U test showed no significant difference in gastric residual volume between the experimental and control groups on the first day (p = 0.910), the second day (p = 0.887), the third day (p = 0.699), or cumulatively during the study period (p = 0.927). Although the differences were not statistically significant, the average gastric residual volume on the second and third days, as well as cumulatively, was lower in the experimental group than in the control group.
Table 2 also reports 95% confidence intervals (CI) and effect sizes (Cohen’s d) for the differences in GRV between groups. The CIs for mean differences across all days and cumulative values crossed zero, indicating no statistically significant differences between the experimental and control groups. The effect sizes were very small (Cohen’s d < 0.20).
Graphic 2. The trend of gastric residual volume (GRV) in both the experimental and control groups across the three study days and the cumulative measurement.
3.3. The Correlation between Confounding Variables and GRV
Table 3 shows that there was no significant relationship between age and gastric residual volume (p = 0.143), with a weak correlation strength (r = 0.296). Similarly, the APACHE II score was not significantly associated with gastric residual volume (p = 0.573), showing a very weak correlation (r = 0.116). In contrast, blood potassium levels were significantly associated with gastric residual volume (p = 0.022), with a moderate correlation strength (r = 0.447).
| Characteristics | Experiment Group (n=26) | Control Group (n=26) |
|---|---|---|
| Age, mean ± SD | 58.08 ±14.05 | 58.81 ± 10.97 |
| Gender, n (%) | ||
| Males | 16 (61.5) | 10 (38.5) |
| Females | 10 (38.5) | 16 (61.5) |
| APACHE II score, mean ± SD | 20.27 ± 7.31 | 17.92 ± 8.80 |
| The usage of catecholamine drugs, n (%) | ||
| Yes | 14 (53.8) | 16 (61.5) |
| No | 12 (46.2) | 10 (38.5) |
| Type of enteral nutrition formula, n (%) | ||
| Formula Standard formula | 4 (15.4) | 5 (19.2) |
| Formula Kidney formula | 2 (7.7) | 3 (11.5) |
| Formula Diabetes formula | 4 (15.4) | 6 (23.1) |
| Other formula | 16 (61.5) | 12 (46.2) |
| Serum potassium level, mean ± SD | 4.45 ±0.78 | 4.17 ± 0.92 |
| GRV | Experiment Group (n=26) | Control Group (n=26) | Z | p value | 95% CI | Effect Size (d) |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| Day I | 111.35 ± 163.92 | 93.65 ± 111.14 | -0.113 | 0.910 | -63.43 to 98.43 | 0.12 |
| DayII | 92.31 ± 110.21 | 118.65 ± 179.53 | -0.142 | 0.887 | -116.78 to 64.10 | -0.17 |
| Day III | 102.69 ± 128.61 | 103.08 ± 143.27 | -0.387 | 0.899 | -76.24 to 75.47 | -0.003 |
| cummulative | 306.35 ± 302.94 | 315.38 ± 342.16 | -0.92 | 0.927 | -176.72 to 158.66 | -0.03 |
| Confounding Variables | GRV | ||
|---|---|---|---|
| N | r | p value | |
| Age | 26 | 0.296 | 0.143 |
| APACHE II score | 26 | 0.116 | 0.573 |
| serum potassium levels | 26 | 0.447 | 0.022 |
| Confounding Variables | N | Mean Rank | p value |
|---|---|---|---|
| Gender | 0.692 | ||
| Males | 16 | 26.33 | |
| Females | 10 | 26.67 | |
| The usage of catecholamine drugs | |||
| Yes | 14 | 16.43 | 0.035 |
| No | 12 | 10.08 | |
| Type of enteral nutrition formula, n (%) | |||
| Formula Standard formula | 4 | 10.25 | 0.447 |
| Formula Kidney formula | 2 | 13.25 | |
| Formula Diabetes formula | 4 | 9.63 | |
| Other formula | 16 | 15.31 |
| Confounding Variables | β | SE | t | Sig. | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.35 | 4.55 | 1.66 | 0.11 | -1.96 | 17.07 |
| Gender | 0.11 | 120.88 | 0.58 | 0.57 | -183.3 | 322.69 |
| Males | ||||||
| Females | ||||||
| APACHE II score | -0.125 | 8.64 | -0.6 | 0.56 | -23.29 | 12.88 |
| The usage of catecholamine drugs | -0.18 | 126.88 | -0.83 | 0.42 | -370.46 | 160.66 |
| Yes | ||||||
| No | ||||||
| Type of enteral nutrition formula, n (%) | 0.36 | 53.50 | 1.78 | 0.91 | -16.86 | 207.1 |
| serum potassium levels | 0.25 | 80.99 | 1.21 | 0.24 | -71.53 | 267.52 |
Table 4 shows that there is no significant relationship between gender and gastric residual volume (p = 0.692) and between the type of enteral nutrition formula and gastric residual volume (p = 0.447). Meanwhile, there is a significant relationship between the use of catecholamine drugs and gastric residual volume (p = 0.035)
Table 5 shows the relationship between age, gender, APACHE II score, use of catecholamine drugs, type of nutritional formula, and serum potassium levels using linear regression analysis. The analysis results showed that all cofounding variables did not significantly influence the effectiveness of the intervention.
4. DISCUSSION
This study aimed to evaluate the effectiveness of intermittent feeding combined with the right lateral position on gastric residual volume (GRV). Although the analysis did not show statistically significant differences, the average GRV on the second day, third day, and cumulatively was lower in the experimental group than in the control group. These findings suggest a possible trend; however, they cannot be interpreted as definitive evidence of effectiveness.
Several previous studies have investigated body positioning during enteral nutrition. One study using a bolus feeding method found that GRV in the right lateral position was significantly lower than in the supine position (p < 0.05), but no similar studies have been conducted using the intermittent feeding method [12]. Another study reported that GRV in patients 10 minutes after receiving normal saline via NGT was lower in the right lateral position [16]. The findings of the present study did not show these significant differences, which may be due to variations in feeding methods, patient populations, and the presence of multiple confounding factors in the ICU setting.
GRV is influenced by several clinical variables, which may have contributed to the results of this study. Factors such as the use of drugs affecting gastric motility, the presence of metabolic disorders, and the number of comorbidities have been associated with higher GRV [17-21]. Specifically, in critically ill patients, vasopressors have been identified as contributing to poor outcomes related to GRV [22-25]. This aligns with the current study, in which more than 50% of patients received catecholamine drugs, and there was a significant relationship between catecholamine use and GRV.
Similarly, serum potassium levels were significantly correlated with GRV, supporting previous studies showing that electrolyte imbalances, particularly hypokalemia, negatively affect gastrointestinal function [26-28]. These results highlight the importance of considering drug exposure and metabolic status when interpreting gastric tolerance during enteral feeding.
In summary, intermittent feeding in the right lateral position did not significantly reduce GRV compared with the semi-recumbent position, although there was a trend favoring the experimental group. The associations observed with catecholamine use and potassium levels emphasize the need to consider the clinical context when evaluating gastric tolerance. Future research with larger sample sizes, better control of confounding factors, and inclusion of broader clinical outcomes is warranted to clarify the potential role of feeding position in critical care nutrition.
5. IMPLICATION FOR NURSING
The results of this study have implications for nursing practice, education, and research. They can inform nursing interventions aimed at meeting patients’ nutritional needs and managing recurrent episodes of increased gastric residual volume, particularly in ICU settings. Positioning patients in the right lateral position may help prevent pressure injuries, reduce gastric reflux, improve cardiac output, and enhance patient comfort. This study contributes to theory development by expanding scientific knowledge on nutritional support and the management of increased gastric residual volume in patients receiving enteral nutrition. Furthermore, the findings can serve as preliminary data for future research on the effects of intermittent feeding combined with the right lateral position on gastric residual volume and other forms of gastrointestinal intolerance, as studies in this area remain limited.
6. LIMITATION
It must be acknowledged that this study has several limitations. First, changes in patient conditions, such as extubation, transfer to another room, emergence of contraindications, or undergoing surgical or diagnostic procedures, prolonged the time required to collect data. Second, the timing of the intervention varied between respondents because it depended on the patient’s ICU admission time, requiring research assistants to be available when the primary researcher was not on site. Third, patients’ moods sometimes affected positioning, as restlessness or resistance necessitated waiting for the patient to calm down before the intervention could be carried out. Fourth, during intermittent feeding using a feeding burette, differences in the administration speed of the nutritional formula could occur, as the rate was controlled manually via the roller clamp.
Additionally, the sample size was relatively small and limited to a single institution, reducing diversity and representativeness. The study also had potential for bias, as neither researchers, research assistants, nor respondents could be blinded to the intervention. Blinding was not feasible due to the nature of the intervention, which made it apparent which respondents received the experimental treatment and which received standard care.
CONCLUSION
This study did not find a statistically significant effect of intermittent feeding combined with the right lateral position on gastric residual volume compared with the semi-recumbent position. Although a positive trend was observed, these results should be interpreted cautiously and cannot yet be considered evidence of clinical benefit. The findings emphasize that gastric emptying and tolerance to enteral nutrition are influenced by multiple factors, including catecholamine use and electrolyte balance.
Further research with larger sample sizes, longer follow-up, and more diverse populations is needed to clarify the potential role of patient positioning in enteral feeding. Future studies should also consider the influence of diagnosis, comorbidities, and sedative use on gastric residual volume.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: P.R.S.: Study conception and design; D.D. and C.E.: Writing-Original draft preparation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ICU | = Intensive Care Unit |
| GRV | = Gastric Residual Volume |
| CONSORT | = Consolidated Standards of Reporting Trials |
| NGT | = Naso Gastric Tube |
| OGT | = Oral Gastric Tube |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has passed the ethical test from the Health HREC of Persahabatan Hospital with letter number 0031/KEPK-RSUPP/02/2024
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
All the data and supporting information are provided within the article.
FUNDING
The study was funded by the Research and Development Center Universitas Indonesia (Grant no: NKB-51/UN2.RST/HKP.05.00/2024).
ACKNOWLEDGEMENTS
The authors deeply thank Persahabatan General Hospital, the nurses, and the patients who participated in this research


