All published articles of this journal are available on ScienceDirect.
Hospital-acquired Pressure Ulcers in Trauma Patients: A Retrospective Study of 410 Patients at a Referral Trauma Center in the North of Iran
Abstract
Background:
Pressure ulcers (PUs) are among the most common chronic ulcers and complications of hospitalization.
Objective:
The present study aims to evaluate the prevalence of hospital-acquired PUs and their grades in trauma patients, comparing demographic characteristics, clinical features, and outcomes among patients without and with PUs referred to a trauma center in the North of Iran.
Methods:
In a retrospective study, 410 patients with trauma referred to a trauma center in the North of Iran were enrolled. Data were collected using a simple random sampling from March 2019 to September 2019.
Results:
The prevalence of PU in patients with trauma was 27.6%. Grade III (35.5%) and grade I (3.5%) wounds had the highest and lowest frequency of PU, respectively (P<0.001). The mean age of patients with PU was higher than patients without PU (61.73 vs. 47.71 years, P<0.001). The mean hemoglobin level of patients with PU was lower than patients without PU (9.93 vs. 12.25, P<0.001). PUs were more common in smokers compared to non-smokers (P<0.001), with a history of PU (P<0.001), a history of diabetes mellitus (P<0.001), and a history of hypertension (P<0.001). The mean length of stay in the hospital for patients with PU was higher than for patients without PU (13.02 vs. 5.54 days, P<0.001). 74.3% of people with PUs were completely immobile (P<0.001), and 60% of them had mild brain damage (GCS of 13 to 15). Also, the number of people with severe and moderate brain injury among PUs patients was 15% and 24.7%, respectively (P<0.001). Mobility, brain damage, Hemoglobin rate and smoking status were risk factors for pressure ulcers.
Conclusion:
Therefore, it is recommended that health managers and policymakers develop care and treatment plans by considering these risk factors.
1. INTRODUCTION
Pressure ulcers (PUs) are among the most common chronic ulcers and complications of hospitalization [1, 2]. PU is a localized lesion on the skin or underlying tissue, which often occurs in hospitalized patients due to pressure or friction on areas of the body, especially bony prominences [3]. PUs based on severity can be classified into four stages: redness of the skin (stage 1), loss of epidermis (stage 2), and loss of fat, muscle, and bone (stages 3 and 4) [4]. PUs are often associated with high treatment expenses, a higher length of stay in the hospital, and a higher risk of infection and mortality for the patients [5-8]. Furthermore, PUs can be good indicators of the quality of patient care, as their incidence varies in different hospitals and health centers [9, 10]. These injuries are often a secondary diagnosis rather than the leading cause of hospitalization, and sadly, they are frequently overlooked by carers [11]. Patients hospitalized or confined to a bed or wheelchair are at high risk for PUs due to clinical instability and less mobility [1, 8, 12]. While other examples of contributing factors for PUs are age, comorbidities, length of stay in hospital, malnutrition, smoking, and prolonged mechanical ventilation [13-17]. The risk of death from a patient with a PU is 2 to 6 times higher than a patient with healthy skin [18]. Although the quality of the care provided has improved significantly in many nations in recent years, nevertheless, PUs remains a significant health challenge worldwide [19]. The prevalence of PUs is varied in different regions of the world [8]. In Iran, for instance, the prevalence of PUs is believed to be between 10 and 50% [19]. According to Akhkand and Karimian studies, the prevalence of PUs in intensive care units (ICUs) ranges between 3.6% and 45.7%, although a more recent study indicated that this rate could be as low as 19% [8, 20]. PUs can cause significant pain, increased risk of infection and sepsis, additional surgical procedures, prolongation of length of stay in the hospital, and imposing additional costs on the patient and the health care unit [8]. Patients with PU often suffer from compromised quality of life and emotional wellbeing [21]. On the other hand, the cost of treatment of PU is 2.5 times the cost of its prevention [22]. Hence, it is necessary to identify high-risk patients and related factors while taking appropriate preventive measures in order to prevent the negative clinical and economic outcomes of PUs [1]. Therefore, the present study aimed to assess the prevalence of hospital-acquired PUs and their grades in patients with trauma, comparison of demographic characteristics, clinical features, and clinical outcomes among patients without and with PUs, and mobility and brain damage among patients with PUs at a referral trauma center in the North of Iran.
2. MATERIALS AND METHODS
2.1. Study design and subjects
In a retrospective study, 410 trauma patients referred to a trauma center in the North of Iran were enrolled. Based on equation 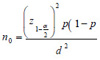 , using the ratio of 0.19 in the previous study [20], d=0.038 and
, using the ratio of 0.19 in the previous study [20], d=0.038 and 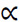 =0.05, the minimum sample size was obtained in 410 cases. Data were collected using a simple random sampling from March 2019 to September 2019. The required number of samples was selected using the table of random numbers and the last three digits of patients' files code. Patients who were admitted for at least 24 hours without PUs were included. The Ethics Committee approved this Research of Guilan University of Medical Sciences (IR.GUMS.REC.1399.072).
=0.05, the minimum sample size was obtained in 410 cases. Data were collected using a simple random sampling from March 2019 to September 2019. The required number of samples was selected using the table of random numbers and the last three digits of patients' files code. Patients who were admitted for at least 24 hours without PUs were included. The Ethics Committee approved this Research of Guilan University of Medical Sciences (IR.GUMS.REC.1399.072).
2.2. Data Collection
Data such as age, sex, length of stay in the hospital, history of PU, degree of PU, smoking, diabetes mellitus (all types of diabetes), blood group, blood pressure, hemoglobin level, mobility, and Glasgow coma scale (GCS) was collected. Skin assessments are generally classified in accordance with the European PU Advisory Panel (EPUAP) classification system for PU as normal or indicative of PU stages (I-IV): I) non-branched erythema; II) loss of skin with partial-thickness; III) loss of full-thickness skin and IV) Loss of full-thickness tissue [23]. Furthermore, the extent of brain damage was estimated based on the GCS criterion (a criterion for determining loss of consciousness in people over five years old):
1. Mild injury (GCS of 13 to 15).
2. Moderate injury (GCS of 9 to 12).
3. Severe injury (GCS of 3 to 8).
2.3. Statistical Analysis
Quantitative and qualitative variables were presented using mean (standard deviation) and number (percentage). The normality of the data was assessed using the Kolmogorov-Smirnov test. Independent t-test and chi-square test were used to evaluate study variables. Multivariate linear regression was applied to identify independent predictors of PU. All probable risk factors were first tested; then, a regular set of independent statistically remarkable covariates were designed in a multivariable model through binary logistic regression, and all data were analyzed two-tailed. A p-value < 0.05 was assumed statistically considerable for the aims of this paper. SPSS® V24.0 statistical package was used for conducting all analyses.
3. RESULTS
3.1. Participants
A total of 410 patients with trauma participated in the present study. Of the participants, 53.7% were male, 24.1% were smokers, 23.7% had a history of diabetes mellitus, and 31% had a history of hypertension. The mean age, hemoglobin level, and length of stay in the hospital were 51.57 (SD=24.67), 11.61 (SD=2.26), and 7.60 (SD=9.83), respectively (Table 1).
3.2. Prevalence of PU and its Grades in Patients with Trauma
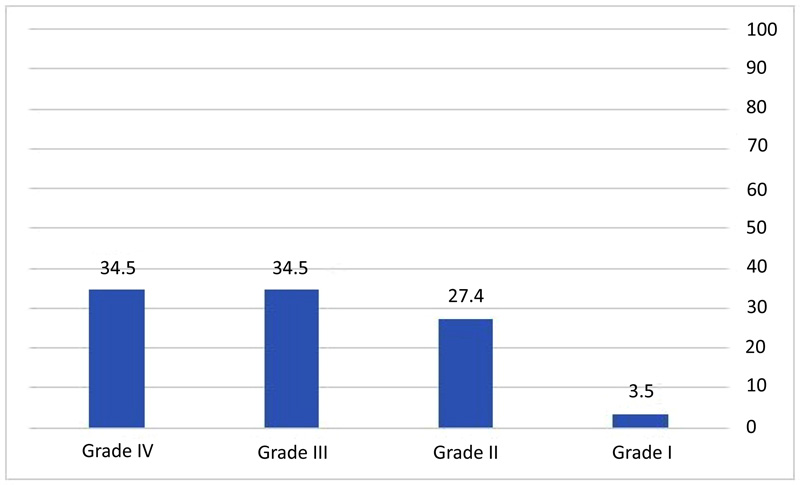
The prevalence of PU in patients with trauma was 27.6%. Grade III (35.5%) and grade I (3.5%) wounds had the highest and lowest frequency of PU, respectively (P<0.001). The frequency of PUs grades in patients with PU is shown in Fig. (1).
3.3. Comparison of Demographic Characteristics, Clinical Features, and Clinical Outcomes among Patients without and with PUs
As presented in Table 1, the mean age of patients with PU was higher than patients without PU (61.73 vs. 47.71 years, P<0.001). The mean hemoglobin level of patients with PU was lower than patients without PU (9.93 vs. 12.25, P<0.001). Also, PU was more common in males than females (P=0.888), smokers compared to non-smokers (P<0.001), with a history of PU (P<0.001), with a history of diabetes mellitus (P<0.001), and with a history of hypertension (P<0.001). The mean length of stay in the hospital for patients with PU was higher than for patients without PU (13.02 vs. 5.54 days, P<0.001).
| Total (n=410) | Patients without PUs (n=297) | Patients with PUs (n=113) | P-value | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Gender | 0.888 | |||
| Male | 220 (53.7) | 160 (53.9) | 60 (53.0) | |
| Female | 190 (46.3) | 137 (46.1) | 53 (47.0) | |
| Age | 51.57 (SD=24.67) | 47.71 (SD=25.23) | 61.73 (SD=19.90) | <0.001 |
| Smoking | <0.001 | |||
| Yes | 99 (24.1) | 55 (18.5) | 44 (39.0) | |
| No | 311 (75.9) | 242 (81.5) | 69 (61.0) | |
| Blood Groups | 0.340 | |||
| A+ | 106 (25.9) | 85 (28.6) | 21 (18.6) | |
| A- | 27 (6.6) | 18 (6.1) | 9 (8.0) | |
| B+ | 68 (16.6) | 46 (15.5) | 22 (19.5) | |
| B- | 22 (5.4) | 14 (4.7) | 8 (7.1) | |
| AB+ | 19 (4.6) | 13 (4.4) | 6 (5.3) | |
| AB- | 8 (2.0) | 5 (1.7) | 3 (2.6) | |
| O+ | 125 (30.5) | 94 (31.6) | 31 (27.4) | |
| O- | 35 (8.5) | 22 (7.4) | 13 (11.5) | |
| Clinical Features | ||||
| A history of PU | <0.001 | |||
| Yes | 66 (16.1) | 0 (0) | 66 (34.5) | |
| No | 344 (83.9) | 297 (100) | 47 (65.5) | |
| A history of diabetes mellitus | <0.001 | |||
| Yes | 97 (23.7) | 58 (19.5) | 39 (34.5) | |
| No | 313 (76.3) | 239 (80.5) | 74 (65.5) | |
| A history of hypertension | <0.001 | |||
| Yes | 127 (31.0) | 80 (27.0) | 47 (41.5) | |
| No | 283 (69.0) | 217 (73.0) | 66 (58.5) | |
| Hemoglobin rate | 11.61 (SD=2.26) | 12.25 (SD=2.09) | 9.93 (SD=1.79) | <0.001 |
| Clinical Outcome | ||||
| Length of stay in hospital (days) | 7.60 (SD=9.83) | 5.54 (SD=5.72) | 13.02 (SD=15.01) | <0.001 |
Data are presented as number (percentage) and mean (standard deviation).
3.4. Mobility and Brain Damage among Patients with PUs
74.3% of people with PUs were completely immobile (P<0.001) (Table 2), and 60% of them had mild brain damage (GCS of 13 to 15). Also, the number of people with severe and moderate brain injury among PUs patients was 15% and 24.7%, respectively (P<0.001) (Table 3).
| Total (n=410) | Patients without PUs (n=297) | Patients with PUs (n=113) | P-value | |
|---|---|---|---|---|
| Mobility | ||||
| Motionless | 134 (32.7) | 50 (16.8) | 84 (74.3) | |
| High restrictions on movement | 37 (9.0) | 12 (4.0) | 25 (22.1) | <0.001 |
| Mild restriction in movement | 39 (9.5) | 35 (11.7) | 4 (3.5) | |
| No restrictions on movement | 200 (48.8) | 200 (67.3) | 0 |
Data are presented as number (percentage) and mean (standard deviation).
| Total (n=410) | Patients without PUs (n=297) | Patients with PUs (n=113) | |
|---|---|---|---|
| Brain damage | |||
| Mild injury (GCS of 13 to 15) | 333 (81.2) | 265 (89.2) | 68 (60.0) |
| Moderate injury (GCS of 9 to 12) | 47 (11.5) | 19 (6.3) | 28 (24.7) |
| Severe injury (GCS of 3 to 8) | 30 (7.3) | 13 (4.3) | 17 (15.0) |
Data are presented as number (percentage).
3.5. Risk Factors Associated with the Occurrence of Pressure Ulcers
All independent variables were analyzed in binary logistic regression with the dependent variable to analyze their association. Among those variables, mobility, brain damage, Hemoglobin rate and smoking status were found to be significant in binary logistic regression and then taken into multivariate analysis (Table 4). For instance, smokers were 3.4 times more likely to develop PU compared to patients who were non-smokers.
| Variable | B | Exp(B) | 95% CI | P-value |
|---|---|---|---|---|
| Smoking status | 1.225 | 3.404 | (1.596,7.263) | .002 |
| Hemoglobin rate | -.385 | .680 | (0.576,0.803) | <,001 |
| Brain damage | .168 | 1.183 | (1.065,1.314) | .002 |
| Mobility | -1.675 | .187 | (0.125,0.280) | <,001 |
| Constant | 4.288 | 72.844 | - | <,001 |
4. DISCUSSION
This study showed that, generally, the prevalence of PU in patients with trauma was 27.6%. Meanwhile, Grade III (35.5%) and grade I (3.5%) wounds had the highest and lowest frequency of PU, respectively. The mean age of patients with PU was higher than patients without PU. The mean hemoglobin level of patients with PU was lower than patients without PU. PU was more common in males than females, smokers compared to non-smokers, with a history of PU, diabetes mellitus, and hypertension. The mean length of stay in the hospital of patients with PU was higher than patients without PU. 74.3% of people with PUs were completely immobile, and 60% had mild brain damage (GCS of 13 to 15). Also, the number of people with severe and moderate brain injury among PUs patients was 15% and 24.7%, respectively. In the present study, PU was observed in 27.6% of patients with trauma. Furthermore, the prevalence of PU varies in previous studies in different countries. The prevalence of PU in Saudi Arabia [24], Greece [25], Spain [7, 26], Turkey [27], Brazil [28, 29], Norway [23], and Iran [30] was 39.3%, 29.6%, 8.1 to 16%, 15.5%, 11 to 13.6%, 14.9%, and 13.4%, respectively. Differences in findings may be caused by the diversity in the features of the study population, data collection processes, inclusion criteria, and various methods of prevention and control of PU in hospitals [1]. Sample heterogeneity may also affect the prevalence of PU and identified risk factors [23]. Consistent with the present study, previous evidence from Norway [23], Iran [31], Saudi Arabia [24], Portugal [32], Greece [7], Brazil [28, 33], and the United States [34], as well as findings from three review studies showed that prolonged length of stay in the hospital was related with an increased risk of PUs [13, 15, 16]. During a prolonged stay in bed, the patient's tissues remain immobile and pressured, which leads to an increased risk of PUs [1]. One study in Iran showed that PUs in cases with muscle paralysis is five times higher than in others. These patients are at a higher risk of PU due to decreased mobility, increased pressure on the underlying tissues, and tissue necrosis [35]. Also, another study in Iran showed that trauma is a significant risk factor for PUs. Because long-term immobility and increased pressure on an area of the body in trauma patients can lead to the development of PUs [31]. Based on the results of this study, age has been identified as one of the risk factors for PU in patients with trauma, which was concordant with previous evidence from Iran [30, 31], Norway [36], Greece [7], Brazil [28], Portugal [32], and the United States [34]. Meanwhile, factors such as decreased mobility, tissue tolerance, skin vessels, and pain perception could also contribute to an increased risk of PU in older people [15, 31]. Nevertheless, two systematic studies have shown that there is inconsistency in the evidence. Factors such as medical complexity, iatrogenic skin lesions, and the presence of underlying disease in older people may affect findings, and lead to varied results [23]. Grade III and IV ulcers had the highest frequency of PU (34.5%), which was consistent with the results of previous evidence from China [37], Belgium [38], Portugal [32], Norway [36], and Iran [20, 31]. Preventive measures taken by hospital staff can reduce PU progression in the first stage, resulting in a lower incidence of grade III and IV wounds [31]. Consistent with the present study results, two studies have also identified smoking as a predisposing factor for patients with PUs [31, 35]. Nicotine in cigarettes prevents the distribution of prostacyclin and causes the capillaries on the skin's surface to contract. Consequentially, the amount of oxidized blood in the tissues is reduced. Also, carbon monoxide and hydrogen cyanide can hinder the healing process of the wound [31]. This study emphasized that diabetic patients are at a higher risk for PU. Previous studies from Iran [31, 35, 39], Norway [36], Greece [7], and a review study [15, 17] have also reported an association between diabetes and PU. Due to the higher prevalence of diabetes in older people, diabetes may be a confounding factor in analyzing the relationship between age and PUs. However, in the current data analysis, both diabetes and older age have been reported as independent risk factors for PUs [23]. Results of this study implicated that there was a significant relationship between hypertension and hemoglobin levels with PUs. People with hypertension and lower hemoglobin levels were more prone to PUs. Consistent with the present study results, previous studies from Iran [40], and India showed a direct correlation between hemoglobin level and PU development in hospitalized patients [41]. Also, two studies [40, 42] showed a direct correlation between hypertension (systolic blood pressure above 130) and PUs [42]. When the risk of developing a PU is established, individual prevention strategies must be implemented. It is recommended that health managers and policymakers develop care and treatment plans with the risk factors associated with PUs in mind. As nurses have a central role in preventing and managing under-pressure areas, they should also be able to identify the risk factors associated with developing PU and take adequate measures to provide appropriate care. Also, based on the components affecting PUs mentioned in this study, software packages for predicting or preventing pressure ulcers can be designed as a PU reduction strategy.
4.1. Limitations
This study had several limitations: it was conducted only in a single center, which may affect the generalizability of the findings of this study; also, no hypotheses were developed before the study. Thus, extreme caution is recommended for interpreting the statistical significance of this study.
4.2. Implications for Nursing Management
Nursing care has a significant impact on developing and preventing pressure ulcers. The results of this study can be considered in the development of nursing management programs. Nurses should be able to assess the risk of developing PU in patients using evidence-based methods or assessment and predictive tools and be aware of the risk factors that increase the likelihood of developing these lesions. It is also recommended that nurses classify PU development or severity patients according to clinical conditions and related risk factors and then take appropriate preventive measures. Controlling blood sugar and blood pressure, monitoring the hemoglobin and nutrition of patients, moving the patient or using a wavy mattress, timely treatment of burn wounds, and infection control should be an essential part of nursing care interventions for patients at PU risk.
CONCLUSION
Overall, the prevalence of PU in patients with trauma was 27.6%. Also, risk factors for hospital-acquired PUs included age, smoking, a history of PU, diabetes mellitus, hypertension, mean hemoglobin, and burn grade. The mean length of stay in the hospital for patients with PU was higher than for patients without PU. 74.3% of people with PUs were completely immobile, whilst this figure was only 60% for those who had mild brain damage. Also, the number of people with severe and moderate brain injury among PUs patients was 15% and 24.7%, respectively. Therefore, it is recommended that health managers and policymakers develop care and treatment plans by considering these risk factors.
LIST OF ABBREVIATIONS
| PU | = Pressure Ulcers |
| ICUs | = Intensive Care Units |
| GCS | = Glasgow Coma Scale |
| EPUAP | = European PU Advisory Panel |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee approved this Research of Guilan University of Medical Sciences (IR.GUMS.REC.1399.072).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All data were obtained from patients during their hospitalization period, so the regional ethical committee waived the need for informed consent.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The datasets are available from the corresponding author [M.S] upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We sincerely thank Mojdeh Esmailzadeh and Armin Fazeli Masuleh, who participated in the preparation of the final version of the article.


