All published articles of this journal are available on ScienceDirect.
Meta-analysis of the Effectiveness of Steroid Pulse Therapy in Treating Patients with Spinal Cord Injury
Abstract
Background:
High-dose steroid injection therapy is effective in reducing anti-inflammation and edema and is often used to treat patients with acute spinal cord injury. To evaluate the effectiveness of steroid pulse therapy and identify the factors that affect its effectiveness in patients with acute traumatic spinal cord injury.
Methods:
A comprehensive literature search of the databases Pubmed, Medline, the Cochrane Central Register of Controlled Trials, Embase, and CINAHL was performed on July 31, 2019, with no language and time limits. For analysis, studies conducted within the last 10 years were included to reflect on the recent trend.
Results:
A total of 3 randomized controlled trials and 5 observational studies with 2418 patients were included in this meta-analysis. High-dose steroid injection therapy was found to have a high effect on patients with acute spinal cord injury. The following factors had a strong influence on the effectiveness of high-dose steroid treatment in patients with acute spinal cord injuries: injury, onset ASIA, onset neurological assessment scales, time to start treatment after injury, age, BMI, and gender.
Conclusion:
It is necessary to accurately assess the scope of spinal injury in the early stages and actively provide nursing interventions to identify and mediate factors affecting the treatment effect.
1. INTRODUCTION
The spinal cord is a central nerve in the spine that can suffer damage due to accidents, diseases, or any injury to the spine [1, 2]. If the spinal cord is damaged, neurotransmitters between the brain and the body are not properly transmitted, resulting in paralysis of movement, sensation, etc [3]. Thus, a spinal cord injury is mainly caused by trauma such as a car accident or crash, which results in loss of movement due to paralysis of the motor nerves below the level of injury, loss of sensation, and abnormality in bladder and bowel movement controlled by the autonomic nervous system [4, 5]. In the United States, there is an annual occurrence of approximately 54 cases of spinal cord injuries per one million people, or about 17,700 new spinal cord injury cases each year [6]. In South Korea, approximately 74,000 people are afflicted with spinal cord injury, and this number is increasing by 2,000 every year [2]. Acute spinal cord injuries are prevalent among socially active age groups, with the average age of occurrence being 40 years. Furthermore, men are more likely to sustain a spinal cord injury than women [3]. Patients with acute spinal cord injuries suffer from incomplete quadriplegia, incomplete hip paralysis, or total limb paralysis, depending on the degree of damage. Paralysis due to spinal injury causes functional problems in various parts of the human body, such as persistent pain, restriction of movement, bowel-nervous disorder, and respiratory failure [4, 7]. Therefore, treating spinal injury in the acute stage is important not only to save the lives of the patients but also to help them maintain a healthy life.
High-capacity steroid therapy has long been recommended for the treatment of spinal cord injury, but its effects are known to be somewhat poor. High doses of steroids have been reported to reduce inflammatory reactions and edema to prevent the deterioration of neurological functions and the progression from incomplete paralysis to complete paralysis [3-15]. However, it has also been reported that the treatment lacks a clear basis; its efficiency is questionable, and the side effects of high-dose steroid treatment cannot be ignored [14, 16-19]. Currently, this treatment is not recognized as a standard treatment, but it is still being applied in clinical practice. Even with side effects taken into account, if high-dose steroid injections are to be applied as a treatment to patients with acute spinal injury in clinical situations, more methodological intervention and observational studies will have to be conducted to control the side effects. There is a lack of randomized controlled trial studies associated with the effects of high-dose steroid injection therapy despite it being the primary choice of treatment for acute spinal cord injuries. Further research needs to be conducted to assess acute spinal cord damage.
1.1. Background
During the first 4-8 hours after spinal cord injury, partial tissue necrosis begins to appear in the impacted area, and within 24-48 hours, tissue necrosis becomes apparent and progresses extensively to the adjacent spinal cord segments that have been impacted. This change reaches its peak at 24 h, following which there are minimal changes in the next 24 h [3, 8]. Spinal cord injury produces two types of damages: primary and secondary. Features of primary damage include the ejection light of electrolytes because of mechanical damage, bleeding, and cell damage, while features of secondary damage include edema, inflammation, ischemia, growth defects, the release of cytokines, reduction in blood flow, calcium accumulation, peroxide glass, etc [9-11].
Since most patients with acute spinal injuries die from breathing difficulties and acute circulatory disorders, it is important to align the treatment of damaged vertebrae with neurological examination during the acute stage to prevent further damage [6]. It is very important to supply oxygen to patients during the acute period as it can reduce secondary ischemic damage to the spinal cord. Patients with spinal cord injuries suffer from interruptions to the cardiac accelerator nerves, affecting the sympathetic neuroanatomy, which result in a decrease in heart rate along with a decrease in cardiac output [12, 13]. Surgery for acute spinal cord injury involves removing structures pressing against the spinal cord and aligning the spine using instruments or bone transplants, which is done when the deformation is not conquered in a non-surgical manner, or if the spine is contaminated with progressive neurological or open wounds [4, 10]. Furthermore, it has been reported that administering large amounts of steroids to patients within 8 hours of damage is effective in recovery [14]. The mechanism of action of steroids is not exactly clear, but it is known to stabilize the cell membrane, neutralize the peroxide, reduce the accumulation of calcium in the cell, decrease the excitant amino acid, decrease the edema of tissue, and increase blood flow to the spinal cord. The guidelines for steroid medication instruct to first inject methylprednisolone (30 mg per kilogram of weight) into the vein for 15 min, followed by 45 minutes of rest, and then injecting 5.4 mg per kilogram of weight per hour over 23 hours for 3-5 days [15-17].
The effects of steroid therapy are unclear for various reasons. First, the positive effect of high-dose steroid injection was reported only in a small number of groups [2]; second, improvements in spinal cord segments did not lead to improvements in survival rate or quality of life [7]; third, a lack of reproducibility of results in other studies; and fourth, its use results in a combination of side effects such as high blood sugar, infection, delayed wound treatment, and gastrointestinal bleeding [18]. There are no reports of getting better results from using steroids for longer than 24 hours in spinal cord damage. Long-term steroid use only increases the risk of complications such as delayed ulcer healing and infection. Thus, attention should be paid to the various side effects of steroid therapy.
Treatment outcomes for acute spinal cord injury vary depending on the location and scope of the damage. Moreover, it is not clear whether a treatment is really effective or what factors affect the treatment because of insufficient prognosis for acute spinal cord injury and its associated factors. Furthermore, the primary and secondary damage mechanisms can proceed more broadly and rapidly in the acute period, therefore various factors of impact need to be identified.
Despite the advantages and disadvantages, clinical trials apply steroid therapy as a primary post-damage treatment for acute spinal cord injury. Therefore, this study aims to analyze the effects of high-dose steroid injection therapy applied to patients with acute spinal cord injuries using meta-analysis and identify the factors related to its effectiveness.
2. THE REVIEW
2.1. Aims
The specific objectives of this study are as follows:
- To quantitatively understand or estimate the effectiveness of steroid pulse therapy in treating patients with acute spinal cord injuries.
- To investigate the effects of factors influencing the effectiveness of steroid pulse therapy in patients with acute spinal cord injuries.
2.2. Review Design
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols [PRISMA-P] 19 will be set as a guidebook for the protocol, and the review methods will be designed based on the meta-analysis of observational studies in epidemiology: a proposal for reporting PRISMA and the Cochrane Collaboration Handbook [18].
2.3. Search Methods
Two independent reviewers (SH and one meta-analysis specialist) selected and reviewed studies. The reviewers independently screened the titles and abstracts of the selected studies to determine whether each citation met the inclusion criteria and assessed eligibility based on a full-text review. The reviewers compared their lists and resolved any differences in opinion through discussion; potential conflicts were resolved with the help of one external specialist who is a nursing science professor. We attempted to plan, perform, and report this meta-analysis in compliance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] [18-25]. Related articles published in English were identified and selected by searching databases of Pubmed, Medline, the Cochrane Central Register of Controlled Trials, Embase, and CINAHL (until July 31, 2019) using the following mesh search terms: “spinal cord/traumatic/acute spinal cord injury,” “steroid/corticosteroid/methylprednisolone/dexamethasone/hydrocortisone/naloxone hydrochloride,” “mega-dose/high-dose/steroid pulse therapy,” “randomized controlled trial/RCT,” and “observational study.” We combined these terms in accordance with the instructions of the database. In addition, the reference lists of retrieved studies and previous reviews and meta-analyses were reviewed and manually searched. No attempt to identify unpublished reports were made. Our search strategies will be peer-reviewed by a second information specialist using the peer-review of the electronic search strategy method
2.4. Study Selection
To select study titles, we first screened identified titles or abstracts and then reviewed the full-text of the articles. Studies were considered eligible if the following criteria were met [1]: had randomized controlled trial design or observational studies involving adult patients [2]; study subjects had undergone high-dose steroid therapy at the time of admission after injuries [3]; had comparison groups of high-dose steroid injection therapy and non-steroid therapy [4]; had reported the factors affecting the effectiveness of steroid pulse therapy, and [5] had mentioned relative outcomes such as related complications.
2.5. Data Extraction
The following data and information were extracted: first author, year of publication, study design, injury levels of spinal cords, time of treatment application, steroid pulse therapy and non-steroid pulse therapy treatment, main outcomes, side effects, factors relating to main outcomes, and study results. The study selection and data extraction were conducted by two authors independently, and discussions were held to resolve disagreements. The following main outcomes were extracted: sensory change and impairment score, which states whether, after applying steroid pulse therapy, the patients suffer from complications such as urinary tract infection, pneumonia, and sepsis. The extracted data and information from studies are presented in Tables 1,2, and 3.
2.6. Quality Appraisal
The Cochrane Collaboration’s “risk of bias [RoB]” and “Risk of Bias Assessment tool for Non-randomized Studies [RoBANS]” were used to evaluate the methodological quality and risk of bias of included randomized controlled trials and non-randomized controlled trials; seven or eight specific domains were examined and measured using this tool: RoB contains domains of sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other” issues. RoBANS contains 6 domains, including the selection of participants, confounding variables, measurement of intervention (exposure), blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. Every domain can be classified as “low risk of bias,” “high risk of bias,” or “unclear risk of bias” in accordance with the judgment criteria (Cochrane Handbook for Systematic Reviews of Intervention. Part 2: 8.5) [26-28].
2.7. Data Synthesis and Analysis
The extracted data were fed into the freeware program Review Manager [RevMan] Version 5.3. Binary outcomes were presented as Mantel–Haenszel style Odds Ratios (ORs) with 95% Confidence Intervals (CIs), and continuous outcomes were reported as inverse variance mean differences [MDs]. A fixed-effect model was adopted in cases of homogeneity [p value of χ2 test > 0.10 and I2< 50%], while a random-effects model was used in cases of obvious heterogeneity (p value of χ2 test < 0.10 and I2 ≥ 50%). Publication bias was evaluated by the demonstration of funnel plots and assessed using the Rosenthal and Rosenberg fail-safe numbers; the latter was weighted by study variance. Fail-safe numbers of less than 5n+10 (where n is the number of studies in the meta-analysis) were considered indicators of publication bias [29].
3. RESULTS
3.1. Literature Search
Computerized and manual searches resulted in 54 citations, 34 of which were excluded because of duplication. From the remaining 20, 7were excluded after reviewing their titles and abstracts. In total, 13 potentially eligible articles were retrieved for full-text review, out of which 5 were excluded. Finally, we included 3 randomized controlled trials and 5 observation studies for synthesized analysis. Detailed review process is presented in Fig. (1).
3.2. Characteristics of the Included Studies
The basic characteristics of the 8 included studies are presented in Table 1. A total of 2418 patients were involved, including 934 for non-steroid pulse therapy, 1484 for steroid pulse therapy (NASCIS-II or III regimen), and the study took place in the US, France, Italy, Japan, and Canada. The sample size ranged from 19 to 1624.

3.3. Quality Assessment
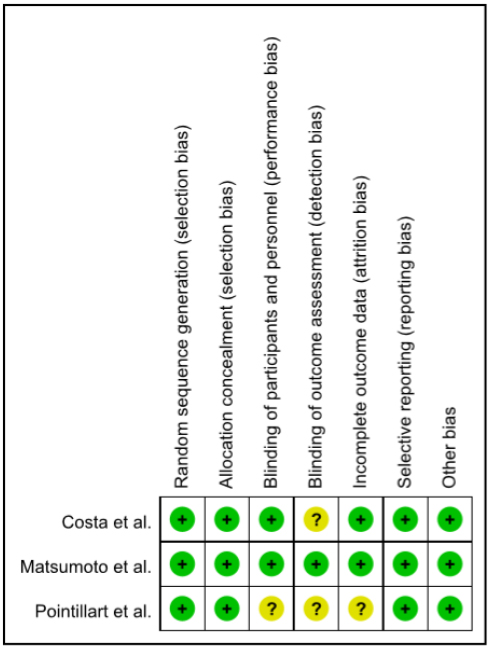
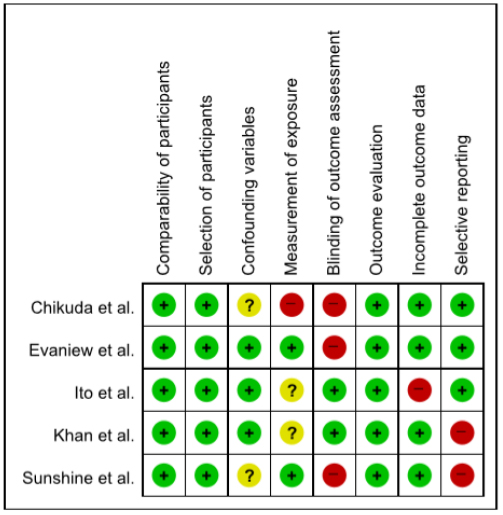
Figs. (2 and 3) illustrate the methodological quality of included randomized controlled trials. Random sequence generation was detailed in all studies (100%), “allocation concealment” was described in all studies (100%), and blindness to “blinding of participants and personnel” in two studies (66.7%). The antecedents of outcome assessments was detailed in one study (33.3%) and “incomplete outcome data” was described in two studies (66.7%). “Selective reporting” was described in all studies (100%). “Other bias” assessed the professionalism of the arbitrator, existence of the intervention manual, and a number of research samples according to the criteria of the previous studies, and was described in three studies (100%), determining that the bias risk was low. In a non-randomized controlled trial qualitative assessment, “comparability of parts” to evaluate selection bias and “selection of parts” were found to be unbiased in all the five studies (100%), while “confounding variables” was less bias risk in all studies. The “Measurement of exposure” of the performance bias, “Blinding of Outcome Assessment” and “Selective reporting” of the detection bias, and “Outcome Evaluation” of the reporting bias were all low in all five studies (100.0%). The “incomplete outcome data” to evaluate attrition bias was found to be highly perverse in only one study. The results of the bias risk assessment for the three randomized controlled trial studies were judged to be low, since more than 50% of these studies were assessed to be low bias in the assessment items except for the cover-up of the “allocation concealment” and “blinding of participants and personnel.” In addition, the five non-randomized controlled trial studies were found to be mostly low in quality assessment other than the “Blinding of Outcome Assessment” items, and were suitable for presenting the findings collectively.
| Authors | Year | Country | Sample Size [N] | Age[mean] | Follow up [month] |
Regimen | Measurement Outcome | ES |
|---|---|---|---|---|---|---|---|---|
| RCT | ||||||||
| Pointillart et al. | 2000 | France | 52 | 43.7 | 12 | NASCIS-II | ASIA | 1.024 |
| Matsumoto et al. | 2001 | Japan | 46 | N/A | 24 | NASCIS-II | ASIA | 0.763 |
| Costa et al. | 2015 | Italy | 19 | N/A | <1 | NASCIS-II | ASIA | 1.012 |
| Observation studies | ||||||||
| Ito et al. | 2009 | Japan | 79 | 46.7 | 3 | NASCIS-II | ASIA | 1.074 |
| Chikuda et al. | 2014 | Japan | 1624 | 53.8 | <1 | NASCIS-II | JCS, CCI | 0.834 |
| Khan et al. | 2014 | USA | 350 | N/A | N/A | NASCIS-II | ASIA | 0.673 |
| Evaniew et al. | 2015 | Canada | 88 | 48.2 | 4 | NASCIS-II | ASIA | 0.841 |
| Sunshine et al. | 2017 | USA | 160 | N/A | 1 | NASCIS-II | ASIA,FIM | 0.910 |
| Categories | k | ES | U3 | 95% CI | RR | I2 | Q | p | |
|---|---|---|---|---|---|---|---|---|---|
| Sensory Loss | 2 | 1.208 | 78.12 | 0.704 | 1.512 | 0.71 | 80.27 | 11.311 | <.001 |
| Neurological scores | 8 | 1.437 | 85.23 | -0.124 | 1.113 | 0.82 | 79.45 | ||
| ASIA | 7 | 1.555 | 88.24 | 0.881 | 1.207 | 0.83 | 75.19 | ||
| Total effect size | 8 | 1.011 | 83.19 | 0.742 | 1.388 | 0.91 | 71.76 | 22.07 | <.001 |
| Categories | k | ES | 95% CI | RR | Q | I2 | |
|---|---|---|---|---|---|---|---|
| Age | 3 | 0.243 | -0.022 | 0.043 | 0.35 | 112.874 | 76.27% |
| Gender | 4 | 0.264 | 0.005 | 0.022* | 0.61 | 81.463 | 74.77% |
| BMI | 2 | 0.318 | 0.038 | 0.068* | 0.64 | 78.572 | 64.12% |
| Onset ASIA | 7 | 0.799 | 0.056 | 0.087* | 0.85 | 2.741 | 22.46% |
| Onset neurological assessment scores | 5 | 0.721 | 0.638 | 0.822* | 0.82 | 35.741 | 36.61% |
| Injury location or levels | 7 | 0.835 | 0.142 | 0.576* | 0.90 | 24.743 | 27.48% |
| Time to start treatment after injury [hours] | 6 | 0.692 | 0.024 | 0.387* | 0.76 | 42.715 | 45.13% |
3.4. Main Analysis
3.4.1. Overall Effect Size of Steroid Pulse Therapy in Patients with Traumatic Spinal Cord Injury
The overall effect size of steroid pulse therapy in patients with traumatic spinal cord injury is shown in Table 2. In this study, effective size, non-protein fraction (U3), and 95% CI were analyzed. I2 tests were conducted to identify heterogeneity [identity] of the individual effect size. Results showed that I2 was 71.76 (p <.001), indicating that individual studies were heterogeneous at the 71.76% level. Therefore, a random effect model instead of a fixed-effect model was used to calculate the size of the effect. The overall average effect size of the steroid pulse therapy was found to be 1.011 that corresponded to the medium effect size according to the effect size analysis criteria proposed by Cohen (1988). The percentiles of non-overlap (U3) were 71.76% in the experimental group when the average score of the control group was 50%, which can be interpreted as an increase of 21.76% over the control group. In addition, the overall average effect size of steroid therapy was statistically significant as the 95% CIwas0.742-1.388 and the p-value was less than .001 with the significance level (alpha) of .05.
3.4.2. Factors Affecting the Effectiveness of Steroid Pulse Therapy in Patients with Traumatic Spinal Cord Injury
The results of an analysis of the average effect size by dependent variables of the effectiveness of steroid injection therapy are shown in Table 3. The Q test was used to test the homogeneity of effect sizes of studies.There was heterogeneity in all factors such as gender (Q=81.463, p<.05), body mass index (BMI) (Q=78.572, p<.05), onset ASIA (Q=2.741, p<.05), injury location (Q=24.743, p<.05), time to start treatment after injury (Q=42.715, p<.05), and onset neurological assessment scores (Q=35.741, p<.05). The analysis using I2, which indicates the ratio of variance between studies, showed that there was heterogeneity in effect size of all factors such as gender (74.77%), BMI (64.12%), onset ASIA (22.46%), injury location (27.48%), onset neurological assessment scales (36.61%), and time to start treatment after injury (45.13%). Therefore, random-effects model was applied to analyze average effect size of variables and there were significant effect of injury location (ES=0.835, 95% CI=0.142~0.576), onset ASIA (ES=0.799, 95CI=0.056~0.087), onset neurolo- gical assessment scales (ES=0.721, 95% CI=0.638~0.822), time to start treatment after injury (ES=0.692, 95% CI=0.024~0.387), BMI (ES=0.318, 95% CI=0.038~0.068), and gender (ES=0.264, 95% CI=0.005~0.022), in the order they have been mentioned.
3.4.3. Analysis of Publication Bias and Reliability for Calculated Effect Size
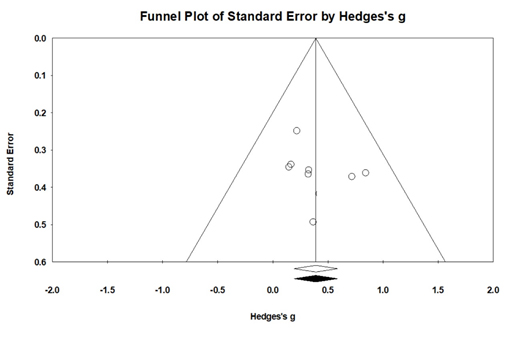
For assessing the potential of publication bias influencing the results of a meta-analysis, the “fail-safe N[Nfs]” were calculated by Rosenthal (1991). The fail-safe N[Nfs] refers to the number of additional “negative” studies (studies in which the intervention effect was zero) that would be needed to increase the P-value for the meta-analysis to above 0.05 (Rosenthal 1979). In the analysis of the fail-safe N using the conventional method, the safety factor value was 153, demonstrating reliability. Finally, when publication bias was suspected, it was re-analyzed using the Duval and Tweedie’s trim-and-fill method. When the trim-and-fill method was applied, there was no newly filled effect size, and the adjusted and observed mean effect sizes were all 0.64, showing no difference. In addition, the 95% CI of the adjusted mean effect size was 0.47-0.85, which was significant. Additionally, the nearly-symmetrical funnel plot suggested that publication bias was unlikely in Fig. (4) (Egger's test, t = 0.90, p > .10).
4. DISCUSSION
The prognosis for spinal cord injury is poor because these injuries can affect the function of the spinal cord below the damaged area and the motor and sensory abilities of many of the areas the spinal cord is responsible for. Although the prognosis for spinal cord injuries vary depending on the range and degree of the injury, it is not well known which other factors have a positive effect on the effectiveness of the treatment [11, 13]. Thus, in this study, the effects of high-dose steroid therapy applied to patients with acute spinal injury were analyzed, and the factors affecting its effectiveness were reviewed through analysis of 6 independently conducted studies.
The effect of high-dose steroid injection therapy on patients with acute spinal injury was measured to the extent of neurological scores and sensory and motor function recovery. The total effect size of the high-dose steroid injection therapy for spinal cord injury patients was 1.011, which is a high effect size according to Cohen (1983) interpretation criteria. In this study, factors affecting steroid injection therapy were suggested, but their effect was interpreted as moderate. Factors affecting the effect size of high-dose steroid injection therapy are, injury location, onset ASIA, neurological assessment scales, time to start treatment after injury, BMI, gender and age (in the order they have been mentioned).
The scope of spinal cord injury showed meaningful relevance to the treatment effect, and the effect size was found to be somewhat large with a value of 1.011. Studies reporting that high-dose steroid injections do not work, have limitations as they do not investigate the degree of spinal cord injury [20], while studies which reported steroid treatment to be slightly more effective classified the degree of spinal injury [21-23]. Therefore, treatment effects need to be assessed by specifically distinguishing the degree of spinal injury. The time to start the treatment after the spinal cord damage was high with an effect size, and showed meaningful relevance to the treatment effect. Steroid therapy is recommended to be applied within 24 hours of spinal cord injury to prevent secondary damage [7, 19, 24]. These results support the validity of the recommended guidelines for high-dose steroid injection in spinal cord injury patients. Onset neurological evaluation scores can be understood in the same context as spinal damage range as factors affecting treatment effects. The wider the scope of spinal injuries, the worse the neurological evaluation score. In the acute stage, neurological outcomes may change rapidly with the onset of damage [8, 11, 19, 25]; therefore, specific assessment timing is needed to assess the therapeutic effects more accurately. Previous studies lacked accuracy when the post-treatment results were measured except before the treatment began. Age is another significant effect factor in this study, implying that even if patients with spinal cord injuries are injected with steroid dosage in accordance with recommended guidelines, the effects may vary depending on age. Research on age-specific resilience needs to be carried out in relation to the curative effects of spinal cord injury patients. In this study, BMI was found to be a factor affecting steroid injection therapy, but the recommended guidelines for steroid injection do not consider BMI. Therefore, it is necessary to further check the patient's BMI to ensure that steroid injections with appropriate doses are being administered. As a factor associated with drug treatment effects in this study, gender is assumed to be meaningful. Steroids are essentially hormones, and the effects of hormone therapy are known to be higher in women than in men [26-28]. Research needs to be carried out to specifically explore steroid effects according to hormonal mechanisms in patients with acute spinal injury. In the future, we hope that the results of this study, along with a variety of other studies, will help evaluate the effectiveness of high-dose steroid injection therapy and will allow us to make more informed decisions.
This study has the following limitations: the variables associated with the side effects of high-dose steroid injection therapy did not meet the minimum number of studies available for analysis and the effect size could not be determined. In the future, studies that shed light on the therapeutic effects of high-dose steroid injection therapy on patients with acute spinal cord injury and the factors affecting its effectiveness will need to be conducted to test the limitations of this study through systematic literature review and meta-analysis.
Nevertheless, this study suggested the effect of high-dose steroid injection therapy on patients with acute spinal injury and the need to study related factors, which could be the basis for the development of evidence-based nursing interventions for the care of these patients.
CONCLUSION
This study was conducted through meta-analysis to identify the effectiveness and associated factors of high-dose steroid infusion applied to patients with acute spinal injury. The results showed that the injury location of spinal cord injuries had the largest effect size, followed by the onset time to start treatment after the injury and the neurological assessment scores. It was also found that to determine the effect of high-dose steroid injection on acute spinal injury patients; it is necessary to accurately assess the scope of spinal injury in the early stages and actively provide nursing interventions to identify and mediate factors affecting the treatment effect. This study is meaningful in that the effect of high-dose steroid therapy for patients with acute spinal injury and its associated factors can be comprehensively identified to suggest the direction of future research related to evidence-based nursing intervention.
CONSENT FOR PUBLICATION
Informed consent was obtained from the respondents.
STANDARDS OF REPORTING
PRISMA guidelines were followed in this study.
FUNDING
This work has supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2020R1F1A1063560).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


