All published articles of this journal are available on ScienceDirect.
Bridging Laboratory and Nursing: A Collaborative Approach to Glucose Monitoring in the ICU
Abstract
Introduction
Capillary glucose testing is commonly used in Intensive Care Units (ICUs) but often yields inaccurate results in critically ill patients due to poor perfusion. Despite Food and Drugs Administration recommendations against its use in this population, it remains widespread. This study, a collaboration between National Reference Laboratory and Cleveland Clinic Abu Dhabi (CCAD), aimed to address these limitations through a structured, nurse-led protocol.
Methods
A four-phase study was conducted: Phase 1: The Siemens RP500 blood gas analyzer was validated against the central lab method (Roche Cobas 6000) to avoid additional venous sampling. Glucose was measured on whole blood already drawn for blood gases. Phase 2: Capillary and whole blood glucose results were compared using the Accu-Chek Inform II and RP500. Phase 3: A structured protocol was implemented in 2019, requiring whole blood confirmation for capillary results ≤4.0 or ≥15.0 mmol/L. Phase 4: Long-term monitoring (2019–2024) assessed compliance, clinical impact, and nursing-led refinements. A 10% allowable difference between paired results was adopted to support bedside decision-making.
Results
The RP500 met performance targets (TAE ≤12.5%) and was accepted as a reference method. Initial analysis showed that 2% of capillary readings fell into high-risk Clarke Error Grid Zones D/E; these were eliminated by whole blood confirmation. Protocol compliance improved from 55% in 2019 to >90% by 2024, with no reported adverse events.
Discussion
Whole blood confirmation at decision thresholds significantly improved result reliability and reduced clinically meaningful discrepancies. The protocol demonstrated sustainability in a high-acuity environment.
Conclusion
The protocol improved glucose testing accuracy and patient safety. It offers a scalable model supported by whole-blood validation, nursing engagement, and practical workflows.
1. INTRODUCTION
Accurate glucose monitoring is essential in critically ill patients to support timely and appropriate treatment decisions. Capillary sample testing on glucose meters/glucometers is widely adopted in ICUs due to its convenience and speed. However, evidence has shown that in critically ill patients, capillary sampling can produce unreliable results due to physiological alterations such as vasoconstriction, edema, and hypoperfusion. These factors compromise sample integrity and introduce significant risks of mismanagement, particularly hypoglycemia or hyperglycemia misclassification.
In recognition of these limitations, the FDA released a safety communication in 2016 advising against the use of capillary glucose measurements in critically ill patients [1]. Despite this, capillary sampling continues to be used, largely due to ingrained workflows and a lack of structured alternatives in clinical practice. The need for a validated, ICU-appropriate approach to glucose monitoring remains a patient safety priority.
Clarke Error Grid (CEG) analysis has long served as a standard method for evaluating the clinical accuracy of glucose monitoring systems [2]. It offers a practical tool for assessing not only analytical performance but also the potential for clinical harm based on the magnitude and direction of measurement error. Although studies have established the greater clinical reliability of venous and whole blood glucose testing over capillary samples in critical care, the adoption of these practices has frequently fallen short of the evidence base.
To address this, the POCT Department at NRL, in collaboration with CCAD, developed and implemented a structured, nurse-led glucose monitoring protocol in 2019, following an initial analytical evaluation of POCT device performance. The protocol required whole blood confirmation for capillary glucose readings ≤4.0 or ≥15.0 mmol/L, aiming to enhance testing accuracy and reduce high-risk clinical decision errors. This initiative also positioned nurses as central to protocol implementation and ongoing quality improvement efforts.
2. METHODS
2.1. Study Design
This prospective quality improvement study was conducted in collaboration between NRL and ICU at CCAD. The study comprised four sequential phases designed to:
• Validate the Siemens RP500 blood gas analyzer as an alternative reference method to the central laboratory, enabling glucose testing from existing blood gas samples and avoiding additional venous draws.
• Evaluate the clinical accuracy of capillary versus whole blood glucose testing using POCT devices (Accu-Chek Inform II and RP500).
• Implement a structured glucose monitoring protocol in 2019, requiring whole blood confirmation for critical capillary values (≤4.0 or ≥15.0 mmol/L).
• Monitor and refine the protocol over a five-year period (2019–2024), assessing long-term compliance, safety outcomes, and the impact of iterative updates led by ICU nursing staff.
2.1.1. Phase 1: Validation of the Siemens RP500 as a Reference Method
To minimize additional venous sampling and utilize existing blood gas draws, the Siemens RP500 blood gas analyzer was validated against the central laboratory reference method—plasma glucose measurement using the hexokinase method on the Roche Cobas 6000 analyzer.This comparison confirmed a Total Allowable Error (TAE) of ≤12.5%, establishing the RP500 as a suitable reference method for whole blood glucose testing in the ICU. This approach enabled efficient evaluation of POCT performance without requiring separate laboratory glucose tests.
2.1.2. Phase 2: Evaluation of Capillary vs. Whole Blood Glucose Testing on the POCT Device
Using paired samples, a three-way comparison was conducted to assess the accuracy of glucose testing using the Roche Accu-Chek Inform II POCT device:
• Capillary blood was collected via fingerstick and analyzed on the Accu-Chek Inform II.
• Whole blood was drawn into a syringe and tested on both:
Accu-Chek Inform II (whole blood mode), and the validated Siemens RP500 blood gas analyzer.
This allowed direct comparisons of:
• Capillary vs. whole blood on the Accu-Chek Inform II
• Capillary Accu-Chek vs. whole blood RP500
• Whole blood Accu-Chek vs. whole blood RP500
Discrepancies between paired measurements were evaluated using Clarke Error Grid (CEG) analysis, which assesses the clinical accuracy of glucose results by plotting each patient’s point-of-care test (e.g., capillary Accu-Chek) against the corresponding reference value (e.g., whole blood RP500). Each pair of measurements is categorized into one of five zones: Zone A (clinically accurate; no effect on treatment), Zone B (benign errors unlikely to affect clinical outcomes), Zone C (overcorrection of acceptable glucose levels), Zone D (failure to detect and treat significant hypoglycemia or hyperglycemia), and Zone E (errors leading to treatment in the wrong direction, with potential for severe harm). By assigning each paired result to a CEG zone, the analysis quantifies both analytical agreement and the potential clinical impact of glucose measurement discrepancies.
2.1.3. Phase 3: Protocol Development and Implementation (2019)
Findings from phase two identified significant inaccuracies in capillary glucose readings at critical thresholds (≤4.0 mmol/L or ≥15.0 mmol/L) when compared to whole blood results. Based on this, a structured protocol was introduced in 2019 requiring mandatory whole blood confirmation for all capillary glucose readings falling within these ranges.
The protocol was co-developed with ICU nursing staff and implemented as a one-year analytic review to evaluate its clinical impact on accuracy, workflow, and safety. Nursing teams were instrumental in the design, application, and iterative refinement of the protocol, with integration into routine ICU workflows.
2.1.4. Phase 4: Protocol Evaluation and Long-term Monitoring (2019–2024)
Following implementation, the structured glucose monitoring protocol was evaluated over a five-year period to assess its sustainability, clinical impact, and integration into routine ICU workflows. Weekly compliance tracking was performed using electronic audit logs, with adherence defined as the proportion of eligible capillary glucose readings correctly followed by whole blood confirmation for values ≤4.0 or ≥15.0 mmol/L.
While initial analytical validation used a Total Allowable Error (TAE) of ≤12.5%, a tighter 10% threshold between paired capillary and whole blood values was adopted to support real-time nursing decision-making. This simpler margin was endorsed by ICU medical and nursing leadership for its practicality at the bedside.
However, applying the protocol to every capillary test proved operationally unsustainable due to concerns around blood wastage, workflow disruption, and redundancy in established hyperglycemia or hypoglycemia cases. Therefore, it was agreed that the protocol would be applied once per 12-hour shift for each patient. If a discrepancy greater than 10% was identified or if the patient exhibited clinical indicators of poor perfusion (e.g., shock, sepsis, edema, or vasoconstriction), whole blood testing was then used for the remainder of the shift.
2.1.5. Key Metrics Monitored during this Period Included
2.1.5.1. Compliance Trends
Sustained improvement through targeted training, real-time feedback, and workflow integration, with rates rising from 53% in 2019 to over 95% by 2024.
2.1.5.2. Good Catches
Cases where high-risk Zone D/E errors were prevented by the protocol were shared during team huddles to reinforce its value.
2.1.5.3. Patient Safety Outcomes
No adverse events related to glucose monitoring were reported over the five-year period.
2.1.5.4. Protocol Iteration
Six refinements were introduced based on frontline nursing feedback, enhancing ownership and ease of implementation.
This long-term monitoring framework balanced clinical accuracy with practical workflow considerations, reinforcing the protocol’s role as a scalable, nurse-led quality improvement initiative in critical care.
2.2. Sample and Statistical Justification
The analytic review included 1,150 glucose results from adult ICU patients in 2019, all of which met protocol criteria. These patients represented a broad spectrum of high-acuity conditions—including sepsis, shock, and respiratory failure—thereby enhancing the generalizability of the findings.
The sample size reflected routine ICU practice rather than a predefined power calculation but exceeded the ≥1,000 results recommended for POCT validation by ISO standards. Clarke Error Grid (CEG) analysis was used to assess clinical accuracy, with a particular focus on Zones D and E, which indicate the potential for inappropriate or harmful clinical decisions
3. RESULTS
3.1. Impact of a Structured Glucose Monitoring Protocol in the ICU
The implementation of a structured glucose monitoring protocol at CCAD led to marked improvements in compliance, accuracy, and patient safety. The protocol was developed following a multi-phase evaluation of glucose testing practices, including the comparison of capillary and whole blood sampling methods, validation of POCT technology, and longitudinal monitoring of protocol effectiveness from 2019 to 2024.
3.1.1. Phase 1: Validation of the RP 500 as a Reference Method
A comparison of glucose measurements from the Siemens RP500 with the central laboratory method demonstrated acceptable agreement. The total allowable error (TAE) between methods was within the predefined threshold of ≤12.5%, with no clinically significant outliers observed. These results confirmed that the RP500 could reliably serve as a reference method for whole blood glucose testing in the ICU setting (Fig. 1).
3.1.2. Phase 2: Evaluation of Capillary vs. Whole Blood Glucose Testing
A three-way comparison was conducted using paired samples (n = 92) to evaluate the accuracy of glucose measurements:
3.1.2.1. Capillary Accu-Chek vs. Whole Blood Siemens RP500
Capillary samples measured on the Accu-Chek Inform II were compared with whole blood glucose results obtained from the Siemens RP500 reference analyzer (Fig. 2). CEG analysis demonstrated that 87% of capillary results fell within Zone A (clinically accurate), 11% in Zone B (minor deviation), and 1% each in Zones D and E, indicating a combined 2% of results with potential for inappropriate treatment decisions or severe clinical risk. No results were observed in Zone C. These findings suggest that although most capillary measurements aligned with the laboratory reference, a small but clinically significant proportion exhibited unacceptable accuracy, raising concerns about the reliability of capillary sampling in critically ill patients.
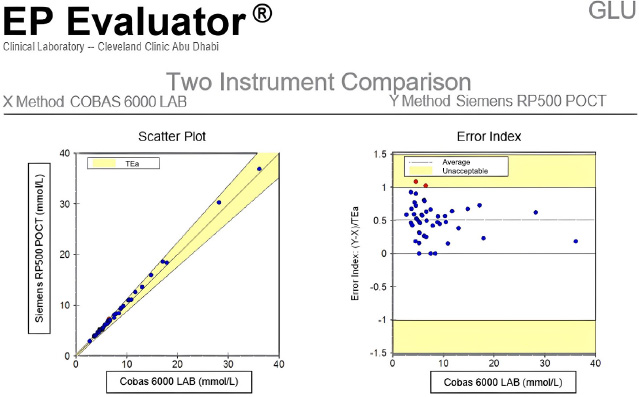
Verification of whole blood glucose testing using Siemens RP 500 blood gas analyzer compared to plasma glucose results on the Roche Cobas 6000 (main laboratory method), demonstrating alignment and validation for ICU use.
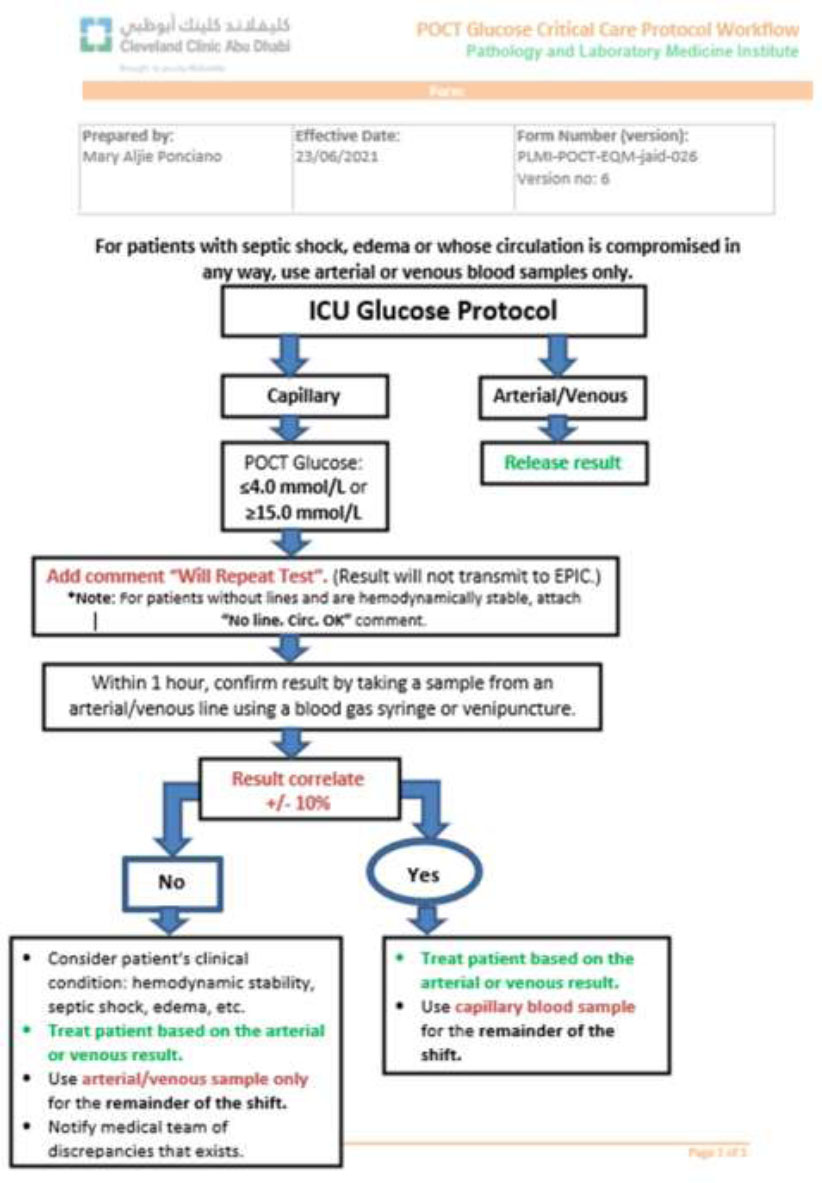
Current version (Version 6) of the ICU glucose monitoring protocol, incorporating nursing feedback, iterative improvements, and real-time operational enhancements to improve compliance and patient safety.
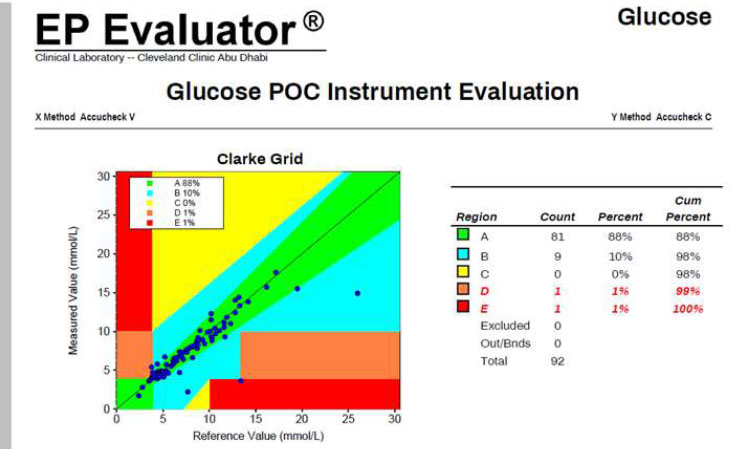
Performance comparison between capillary and whole blood glucose testing using the Accu-Chek Inform II POCT device, highlighting sample type variability and the importance of confirming critical capillary results.
3.1.2.2. Capillary vs. Whole Blood on Accu-Chek Inform II
A direct comparison of capillary and whole blood glucose measurements performed on the same Accu-Chek Inform II device was conducted to evaluate the performance of the point-of-care device using different sample types (Fig. 3). CEG analysis showed that 88% of results fell within Zone A, 10% in Zone B, and 1% each in Zones D and E, with no results in Zone C. These findings closely mirrored those observed in the comparison with the RP500 reference analyzer, indicating that whole blood sampling on the Accu-Chek Inform II provides results suitable for clinical use (Fig. 2).
3.1.2.3. Whole Blood Accu-Chek vs. Whole Blood Siemens RP500
Whole blood results from the Accu-Chek Inform II showed excellent agreement with the RP500 blood gas analyzer, with 99% of values falling in Zone A and 1% in Zone B. Additionally, this comparison demonstrated a Total Allowable Error (TAE) of ≤12.5%, supporting the interchangeability of the two methods for ICU use (Fig. 4).
These findings confirmed that capillary testing, particularly at extreme glucose values, may lead to clinically significant errors. In contrast, whole blood testing via POCT devices or blood gas analyzers provided reliable, plasma-equivalent results suitable for decision-making in critical care.
3.1.3. Phase 3: Protocol Implementation and 2019 Performance Review
Following the capillary sample analysis, a structured ICU glucose monitoring protocol was introduced in 2020, requiring whole blood confirmation for capillary glucose values ≤4.0 mmol/L or ≥15.0 mmol/L.
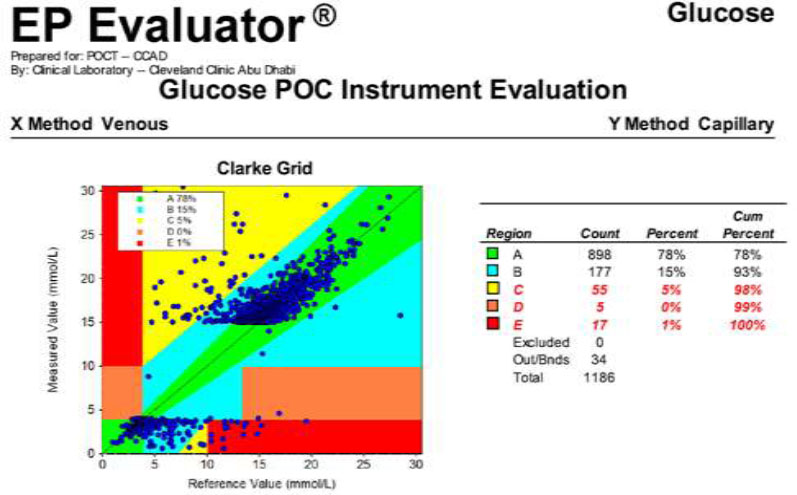
To assess baseline performance prior to full implementation, 1,186 capillary glucose readings from 2019 were evaluated using CEG analysis (Fig. 5):
• Zone A (Clinically Accurate): 898 readings (78%)
• Zone B (Clinically Acceptable): 177 readings (15%)
• Zone C (Unnecessary Intervention): 55 readings (5%)
• Zone D (Inappropriate Treatment): 5 readings (<1%)
• Zone E (Severe Harm): 17 readings (1%)
In total, 22 readings (2%) fell into Zones D and E—representing high-risk errors with potential for clinical harm. These data reinforced the need for a confirmatory protocol using whole blood testing in critical value ranges.
3.1.4. Phase 4: Protocol Evolution and Long-term Impact (2019–2024)
3.1.4.1. Compliance Trends
Initial protocol adherence was 53% in early 2019, gradually increasing to 85% by year-end. Sustained improvements were achieved through targeted education, workflow integration, and nursing engagement. From 2021 onward, compliance consistently exceeded 90%, reaching over 95% by 2024 (Fig. 6).
3.2. Clinical Impact and Safety Outcomes
3.2.1. Good Catches
Routine compliance monitoring identified multiple instances where the protocol prevented Zone D/E errors. These were shared with ICU teams to reinforce the protocol’s clinical relevance.
3.2.2. Error Reduction
Following implementation, Zone E and D errors were eliminated, and Zone D errors were significantly reduced.
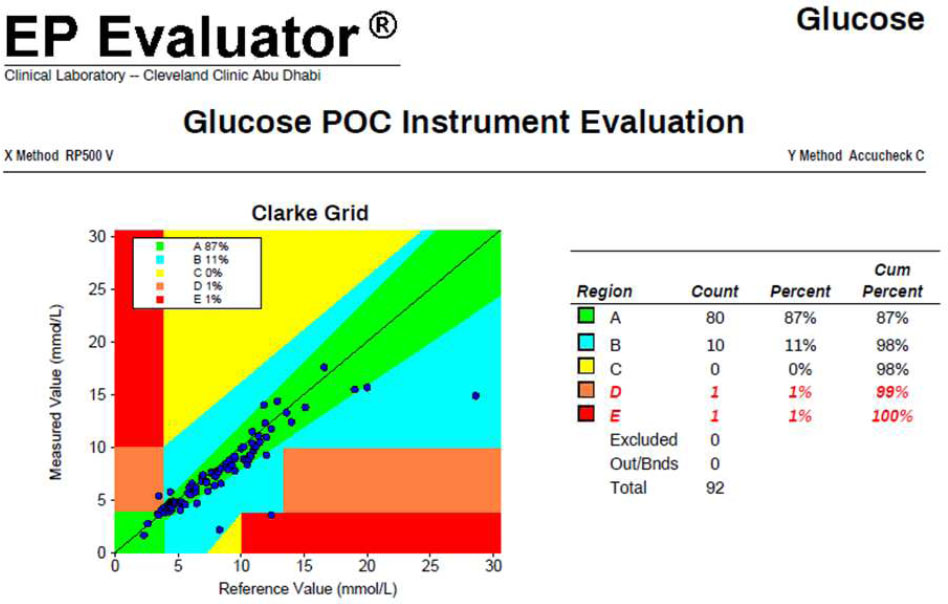
Comparison of capillary glucose readings from the Accu-Chek Inform II versus whole blood glucose results from the Siemens RP 500. This analysis highlighted discrepancies that informed the development of the ICU glucose protocol.
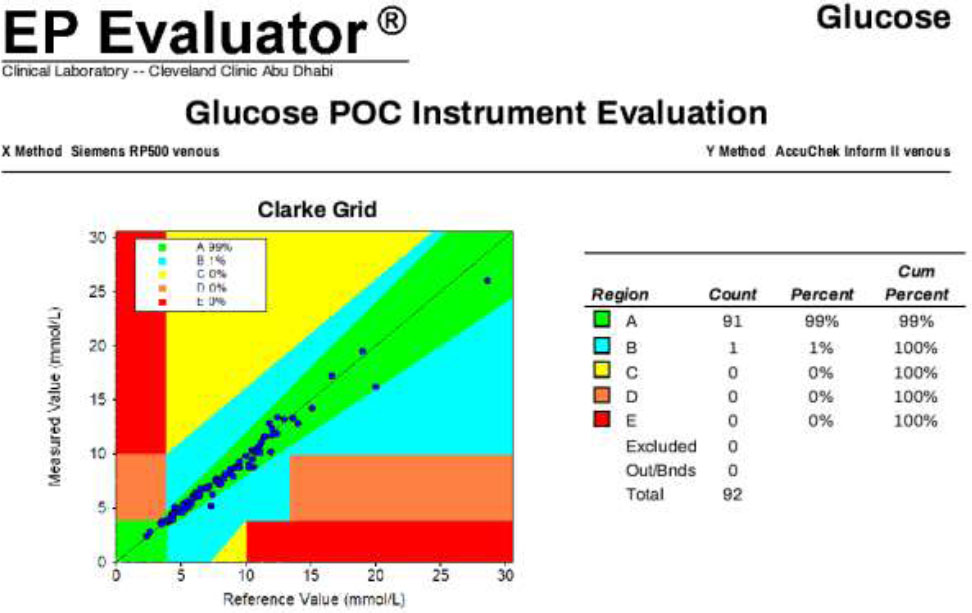
Direct comparison between whole blood glucose results obtained from the Accu-Chek Inform II POCT device and the Siemens RP 500 blood gas analyzer, confirming the accuracy and suitability of whole blood testing on POCT devices.
Clarke Error Grid analysis of glucose results collected in 2019 after the protocol implementation, illustrating the clinical accuracy of capillary glucose testing when used in conjunction with whole blood confirmation.
3.2.3. Adverse Events
No patient harm events related to glucose monitoring were reported over the five-year period.
The implementation of a structured glucose monitoring protocol significantly reduced the risk of high-impact errors associated with capillary testing in the ICU. By mandating whole blood confirmation for critical capillary values and validating POCT technologies for reliability, the protocol enhanced clinical safety, improved compliance, and demonstrated long-term sustainability. These outcomes highlight the value of interdisciplinary collaboration and continuous monitoring in optimizing point-of-care practices in high-acuity settings.
4. DISCUSSION
4.1. The Role of Structured Protocols in Enhancing Patient Safety
The implementation of a glucose monitoring protocol at CCAD underscores the transformative potential of structured workflows in reducing risks associated with capillary blood glucose (BG) testing (Fig. 7). This protocol prioritized critical thresholds (≤4.0 mmol/L and ≥15.0 mmol/L), ensuring readings outside these ranges were validated using whole blood samples. This approach mitigated the inaccuracies of capillary sampling in critically ill patients, significantly reducing the risk of adverse events. Notably, Zone E errors—identified by the Clarke Error Grid [2] as having the highest potential for harm or death—were eliminated through mandatory whole-blood confirmation.
The integration of whole blood testing on the POCT device provided the foundation for introducing a practical, monitorable protocol. Middleware-enabled data capture ensured thorough compliance tracking, with flagged capillary results requiring whole blood confirmation before being documented in the medical record. If the whole blood results fell within 10% of the capillary reading [3], the capillary sample's suitability was validated. However, discrepancies exceeding 10% prompted subsequent glucose testing to rely solely on whole blood samples, enhancing patient safety and result reliability.
Structured protocols also play a vital role in creating consistent clinical workflows. Prior to the protocol's implementation, variability in how nurses interpreted capillary glucose results led to inconsistencies and potential safety risks. Standardizing practices reduced ambiguity, improved accuracy, and allowed nurses to concentrate on providing high-quality care. This approach aligns with global recommendations, including the World Health Organization's (WHO) emphasis on eliminating avoidable harm in healthcare through structured action plans and evidence-based interventions [4-6].
Furthermore, structured protocols are critical in ensuring adherence to professional guidelines. The American Nurses Association (ANA) highlights the role of standardized practices in nursing to support patient-centered care and minimize clinical errors [7]. Similarly, the National Institute for Health and Care Excellence (NICE) stresses the importance of infection control and structured workflows to improve patient safety outcomes [8]. Protocols like those implemented at CCAD exemplify these principles, demonstrating their applicability to complex care environments.
Finally, standardized protocols align with recommendations from the Agency for Healthcare Research and Quality (AHRQ), which underscores the value of using checklists and workflows to enhance consistency and patient safety in clinical settings [9]. By incorporating these structured measures, the glucose monitoring protocol provides a replicable framework for enhancing safety, reducing errors, and promoting consistent nursing practices.
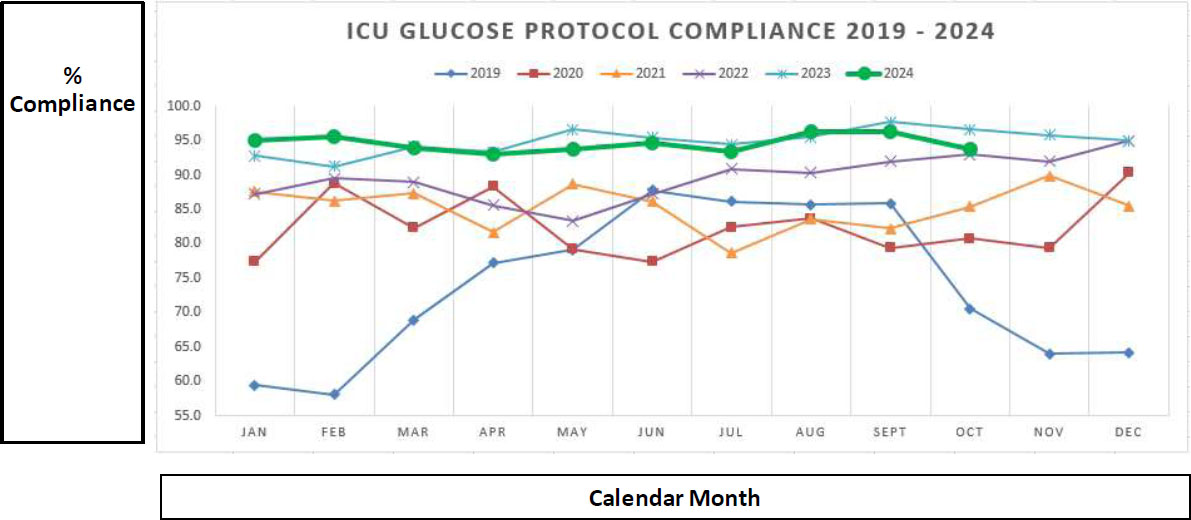
ICU glucose protocol compliance data (2019–2024), demonstrating improved adherence over time driven by nursing engagement, enhanced training, and protocol refinements. Data extracted from POCT middleware and reported to ICU leadership weekly.
| Version | Date | Summary of Changes |
|---|---|---|
| V1 | 18 April 2017 | Initial version of the ICU glucose monitoring protocol. |
| V2 | 17 May 2017 | Minor updates: added document number and corrected a typing error. |
| V3 | 4 September 2018 | Added instructions for patients with septic shock, swelling (edema), or on total parenteral nutrition (TPN). |
| V4 | 22 December 2019 | Cleaned up formatting and removed outdated instructions from Step 4 for better clarity. |
| V5 | 2 March 2020 | Major revision that significantly improved compliance: • Nurses could now select the sample type (capillary, arterial, venous) directly on the device before testing — eliminating the need to manually type this after the test (which was often missed). • The protocol no longer required action on every individual capillary test — only once per shift when readings exceeded limits. • If there is a >10% difference between capillary and arterial/venous results, only arterial/venous samples should be used for the rest of the shift. |
| V6 | 23/06/2021 | Further refinements made in response to feedback from the nursing team to improve usability and clarity. |
| - | Not Important Very Important | Weighted Average | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Training and follow up from Unit Based Educators | 1 (5%) | 0 (0%) | 3 (15%) | 6 (30%) | 10 (50%) | 4.20 |
| Feedback from nursing implementing the protocol | 0 (0%) | 0 (0%) | 3 (15%) | 5 (25%) | 12 (60%) | 4.45 |
| Morning Huddles | 1 (5%) | 3 (15%) | 6 (30%) | 3 (15%) | 7 (35%) | 3.60 |
| ICU manager follow up and feedback | 0 (0%) | 1 (5%) | 5 (25%) | 5 (25%) | 9 (45%) | 4.10 |
| Weekly compliance reports and follow up by managers | 0 (0%) | 1 (5%) | 6 (30%) | 5 (25%) | 8 (40%) | 4.00 |
| Changes/improvements to ICU protocol over time | 0 (0%) | 0 (0%) | 2 (10%) | 7 (35%) | 11 (55%) | 4.45 |
4.2. The Power of Interdisciplinary Collaboration and Engagement
One of the most significant contributors to the protocol’s success was the collaborative effort between CCAD ICU nursing staff and the NRL POCT Department. Nurses, as frontline providers, played a critical role in identifying workflow inefficiencies and suggesting practical modifications during the protocol’s six iterations. This interdisciplinary teamwork aligns with the American Nurses Association's emphasis on collaboration as an essential element of nursing practice, fostering patient-centered care and improving clinical outcomes (10). This collaboration and feedback led to multiple iterations of the protocol, improving compliance further (Tables 1 & 2).
Research supports the value of interdisciplinary collaboration in driving quality improvements. For example, structured interdisciplinary rounds have been shown to enhance communication among healthcare teams, leading to coordinated care plans and better patient outcomes [10). Similarly, interprofessional education and collaborative practice have been identified as key strategies to improve healthcare delivery and patient satisfaction [11]. The iterative refinement of the glucose monitoring protocol at CCAD reflects these principles, with feedback loops (laboratory & nursing) and joint problem-solving sessions ensuring continuous improvement.
For example, early compliance data revealed challenges in manually entering the source of the specimen (e.g., arterial/venous). Collaboratively, the NRL POCT Department implemented a firmware upgrade for the device, introducing a mandatory pre-selection feature for specimen types prior to testing. This upgrade streamlined workflows and significantly improved adherence to the protocol. Weekly compliance reports further strengthened collaboration by identifying areas of non-compliance, which were reviewed and addressed in joint sessions. These reviews promoted shared accountability and empowered nurses to take ownership of patient safety initiatives.
By embedding a culture of accountability and collaboration, the laboratory & nursing team ensured that the protocol achieved sustained success. This experience highlights the transformative power of interdisciplinary teamwork in advancing patient safety and driving sustainable quality improvements.
4.3. The Role of Nursing Leadership
Leadership was central to the protocol’s success. Nurse managers and senior nursing staff played pivotal roles in advocating for adherence to the protocol, mentoring junior staff, and reinforcing its importance in improving patient outcomes. Transformational leadership, characterized by inspiring and motivating teams, fosters a positive work environment and promotes collaboration to achieve shared goals [12]. By employing this leadership style, nursing leaders were able to drive compliance and create a culture of accountability within the ICU.
Regular nursing team huddles were conducted to review protocol adherence trends, share success stories, and identify areas needing improvement. These meetings cultivated a sense of shared responsibility among staff and reinforced the importance of their contributions to patient safety. Effective nursing leaders acted as intermediaries between frontline staff and the NRL POCT Department, ensuring that feedback from nurses helped update the protocol. This collaborative approach aligns with the principle of empowering nurses to take an active role in decision-making, which is essential for achieving sustainable quality improvements [13].
The leadership approach also emphasized a non-punitive environment for addressing instances of non-adherence. By focusing on understanding barriers and providing targeted support rather than assigning blame, nursing leaders-built trust and openness among staff. This approach mirrors the essence of nursing leadership, which involves guiding teams through challenges while fostering confidence and resilience [14, 15]. Ultimately, the nursing leadership at CCAD exemplifies how effective guidance and mentorship can drive the success of complex protocols.
4.4. Shared Governance (laboratory & nursing) and the Role of Magnet Accreditation
The implementation and success of the glucose monitoring protocol at CCAD exemplify the principles of shared governance, a foundational element of the Magnet Recognition Program®. Magnet accreditation highlights the importance of empowering nurses to actively participate in clinical decision-making, fostering a culture of collaboration, accountability, and shared responsibility [14].
Through shared governance, ICU nursing were instrumental in identifying workflow inefficiencies, offering practical suggestions, and ensuring adherence to the newly developed protocol. This approach aligns with the Magnet framework, which emphasizes the significance of nursing leadership and interdisciplinary teamwork in advancing patient care quality and outcomes [15]. By integrating frontline staff into the decision-making process, the hospital created an environment where continuous quality improvement and evidence-based practices became central to nursing activities.
Moreover, the iterative development of the glucose monitoring protocol underscores the role of structured nursing leadership in driving clinical excellence (Fig. 4). Magnet accreditation serves as a guiding framework for fostering innovation, enabling nurses to engage in meaningful contributions to patient care and organizational growth [16-19]. As demonstrated at CCAD, shared governance empowers nursing & laboratory staff to bridge gaps between clinical practice and policy, reinforcing their role in achieving and sustaining quality outcomes.
4.5. Barriers and Challenges
4.5.1. Staff Onboarding and Training
A significant initial challenge involved onboarding new nursing staff and ensuring their familiarity with the glucose monitoring protocol. Given the high turnover rate commonly seen in ICU settings, maintaining consistent compliance necessitated ongoing education and reinforcement. Early in the implementation phase, compliance rates were as low as 53% (Fig. 7), partly due to gaps in staff understanding of the protocol's rationale and execution.
To address this, targeted training sessions were introduced, focusing on both the theoretical and practical aspects of the protocol. Simulated scenarios, such as managing critical thresholds in high-pressure situations, were particularly effective in building staff confidence. Feedback loops were also established, allowing nurses to voice their concerns and seek clarification.
4.5.2. Operational Challenges with Whole Blood Testing
The vast majority of ICU patients have indwelling lines (e.g., intravenous feeds or infusions), which facilitate the collection of whole blood samples following appropriate dead space removal. However, whole blood testing poses logistical challenges compared to capillary testing. Nurses initially faced difficulties accessing arterial or venous samples in patients with poor vascular access or in those without existing lines. Additionally, the time required for whole blood confirmation was perceived as a potential workflow disruption in the high-acuity ICU environment.
To address these challenges, the protocol was designed to streamline testing while maintaining clinical accuracy. Whole blood testing is only applied once per 12-hour nursing shift if initial capillary glucose results are ≤4.0 mmol/L or ≥15.0 mmol/L or if there is a significant discrepancy (greater than 10%) between the capillary and whole blood samples. If capillary results fall within acceptable thresholds and align with whole blood measurements, further whole blood confirmations are not required during the shift.
This approach ensures that potential deteriorations in patient perfusion are identified promptly while minimizing unnecessary disruptions to workflow. The protocol strikes a balance between accuracy and practicality, reinforcing the critical role of whole blood testing in glycemic management within the ICU setting.
4.5.3. Sustainability and Scalability
The protocol’s success at CCAD underscores its potential scalability to other ICU settings and high-acuity departments. By limiting whole blood testing to critical thresholds, the protocol minimized workflow disruptions while ensuring safety. This targeted approach is replicable in resource-constrained environments where routine whole-blood testing may not be feasible.
Additionally, the protocol’s adaptability makes it suitable for diverse clinical contexts. For example, it could be tailored for use in step-down units or emergency departments, where rapid glucose monitoring is equally critical. The focus on compliance monitoring and interdisciplinary collaboration further enhances its transferability to other institutions.
4.5.4. Key Metrics for Success
4.5.4.1. Reduction in Zone E Errors
The elimination of Zone E errors represents one of the protocol’s most significant outcomes. These errors, categorized by the Clarke [9] Error Grid as having the highest clinical risk, were entirely mitigated through mandatory whole blood confirmation for critical capillary readings. This outcome highlights the protocol’s effectiveness in addressing specific limitations of capillary testing in ICU patients.
4.5.5. Broader Implications for Nursing Leadership
The protocol offers valuable insights for nursing leaders aiming to implement evidence-based practices in critical care. Key takeaways include:
4.5.5.1. Feedback Loops
Regular feedback sessions between nurses and the POCT Department were instrumental in identifying barriers and driving improvements.
4.5.6. Educational Implications
Education was a cornerstone of the protocol’s success. Beyond initial onboarding, ongoing training sessions reinforced key principles and addressed emerging challenges. Simulated scenarios, case-based learning, and real-time feedback enhanced staff confidence and competence.
The protocol also highlighted the need for nursing curricula to include comprehensive training on POCT and its limitations. By equipping nurses with a deeper understanding of these tools, educational programs can better prepare them for the complexities of critical care.
5. LIMITATIONS
This study has several limitations. First, the findings are specific to the CCAD ICU population and may not generalize to other clinical settings or patient populations. Second, while the protocol effectively addressed inaccuracies in capillary glucose testing, its implementation required significant training and workflow adjustments, which may not be feasible in resource-limited settings. Third, the reliance on whole blood testing for critical values, though effective, may increase sample collection burden and workflow complexity. Further research is needed to evaluate the protocol's scalability and long-term sustainability across diverse healthcare environments.
CONCLUSION
This study underscores the pivotal role of laboratory and nursing in the development and implementation of a structured protocol that significantly improved glucose monitoring accuracy and patient safety for critically ill ICU patients. By addressing the limitations of capillary testing, fostering interdisciplinary collaboration, and emphasizing accountability, the protocol achieved sustained compliance and reduced high-risk errors. Nursing & laboratory were instrumental in executing the protocol, ensuring its integration into daily workflows, and maintaining its effectiveness over time.
The use of whole blood testing on the POCT device Accuchek Inform II, validated as providing plasma-equivalent results consistent with the laboratory gold standard, was central to the protocol's success. Semi-annual revalidation of this method further ensured its reliability, making it a practical and safe alternative to plasma testing in ICU settings. Nurses’ active involvement in sampling and real-time analysis was crucial to achieving these outcomes.
Notably, To our knowledge, this is the only bespoke protocol specifically designed to assess the clinical suitability of capillary blood sampling in critically ill patients. This unique approach serves as a valuable model for healthcare organizations seeking to address similar challenges. By integrating validated whole blood testing into routine workflows, the protocol not only enhanced glucose monitoring accuracy but also advanced nursing practices, reinforced patient safety, and demonstrated scalability for other high-acuity care environments. Nurses’ feedback and expertise were key to its success, further highlighting their essential role in driving innovation and improving care standards.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: J.H.: Writing paper; L.A.W., M.P., J.T., C.M.: Writing, reviewing and editing. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| TAE | = Total Allowable Error |
| CEG | = Clarke Error Grid |
| CCAD | = Cleveland Clinic Abu Dhabi |
| ICUs | = Intensive Care Units |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study is approved by the CCAD Research Ethics Committee (REC Number: A-2020-079).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


