All published articles of this journal are available on ScienceDirect.
Forecasting Delivery Time of Low-Risk Pregnant Women by Applying Linear Regression
Abstract
Background
The period of normal childbirth is the shortest, lasting no more than 24 hours, but it is the most important because up to 1 in 3 fetal deaths occur during birth. Accurate predictions of the time of birth can help health professionals provide effective care for the women during the time they give birth.
Objective
The aim of this research is to investigate the influence of cervical dilatation, the effacement of the cervix, station of the presentation, body mass index, maternal height, fetal weight, dose and duration of oxytocin exposure, and parity in low-risk pregnant women and to create a mathematical equation model for use in predicting the time to delivery.
Methods
This study is a retrospective descriptive study conducted from July 2023 to December 2023 at Thammasat Hospital. One hundred and eight low-risk pregnant women who had 37+ 0 to 41+ 6 weeks of gestation were selected by stratified random-sampling technique and systematic random sampling technique. The sample size was 108 participants. The research tool consisted of observation sheets and questions. Data analysis was obtained using multiple linear regression with the Stepwise regression method to examine the factor that influenced the time to delivery and create the equation.
Results
The obtained model had an R2 value of 0.316. The significant variables that mostly influence the time of delivery were the timing of oxytocin exposure (β = 0.31, p < .01) and cervical dilatation (β = -31.51, p < .01). The explanatory power of the regression model was statistically significant at 31.03%.
Conclusion
This study was designed for improving the prediction of time to delivery, which can be useful for enhancing the preparation pathways of normal childbirth. In this way, multiple regression analysis showed that the timing of oxytocin exposure and cervical dilatation can predict the time of birth.
1. INTRODUCTION
There are significant differences in labor patterns, and both rapid and slow labor are associated with issues [1-4]. Pregnancy to pregnancy varies in the length of labor. Labor typically lasts 12 to 18 hours during the first pregnancy; subsequent labors are much shorter, lasting 6 to 8 hours on average [5]. Modern obstetrics actively manages labor to prevent protracted labor and its consequences. Furthermore, because the first stage of labor is often the longest [6], healthcare providers have difficult decisions when a laboring woman reaches the second stage for pushing and delivery [7]. Pregnancy-related variables like body mass index, height, and fetal weight have changed over time, according to recent studies [8-10]. It was discovered that pregnant women today have different values from those in the past, which could have an impact on the delivery process, including predicting the time of delivery.
Numerous studies have looked at the overall duration of the labor phases or stages, as well as the median dilation time measured is an integer of 1 cm [4, 6, 10]. Regarding the meanings of the onset of labor and the transition from the latent to the active phase, there is, nevertheless, minimal agreement [11, 12]. The average rate of cervical dilation seems to increase over time without obvious points of acceleration or deceleration, which raises doubts about the shape of the traditional Friedman curve [12, 13]. It is challenging or impossible to pinpoint the precise moment of active labor due to the gradual shift that occurs between the latent and active stages. Average labor curves and tables that show the median traverse time in centimeter integers can help diagnose abnormally slow labor progression. However, these tools are not intended to estimate the time to completion of cervical dilation based on the cervical opening and other factors, such as maternal age [14], height [9], body mass index (BMI) [8], parity [6], gestational age [15], and birthweight [10], which are known to be associated with the length of labor. The length of labor has also been linked to the date of admission to the delivery unit [16], the administration of oxytocin [17], and epidural analgesia [15, 18]. A clinical approach that combines early oxytocin and amniotomy [19]—rather than amniotomy alone [20]—is linked to a marginally shortened hospital stay to birth. Healthcare professionals must make crucial decisions when a laboring woman enters the second stage of labour and is about to deliver because the first stage of labor is typically time-consuming [21]. However, not much work has been done to develop strategies to forecast the likely timing of the transition based on cervical dilatation and other factors.
There is little research that has looked at the time of birth prediction. Not much work has been done to develop strategies to forecast the anticipated timing of the transition based on cervical openness and other factors. The purpose of this study was to study the results of cervical dilatation, the station of the presentation of the baby, body mass index, maternal height and estimate fetal weight at the time of delivery in women with low-risk pregnancies women, so that midwives can use them in caring for births to have more efficiency.
2. MATERIAL AND METHODS
This study was a retrospective descriptive cross-sectional study. Clinical information was retrieved from the medical records of the women who gave birth at Thammasat Hospital in the year 2022-2023 fiscal year. The data collection was conducted from July 2023 to December 2023.
The power analysis was analyzed to confirm that the sample size is sufficient to control Type I and Type II error by setting the effect size value to control the size of the relationship between the independent and the dependent variable from the formula
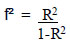 |
Cohen [22], determined the values that influence the determination of effect size for use in Power Analysis used with statistics for multiple regression analysis into 3 sizes as follows: small R2 = 0.02, medium size R2 =0.13, large size R2 = 0.35 in order to control for small errors in the research. At an acceptable level, therefore, the value R2 = 0.13 was used, which can be calculated by substituting the values according to the formula:
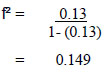 |
The effect size value was obtained equal to 0.149.
Then use the obtained effect size values to calculate the sample group. Polit's sample size calculation formula [22], is as follows:
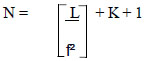 |
When various symbols represent the following meanings:
N = number of samples
L = specific table value for multiple regression analysis based on the number of
variables with alpha value at level 0.05 and Power = 0.80
f2 = Effect Size
K = number of predictor variables
Substitute the values according to the formula
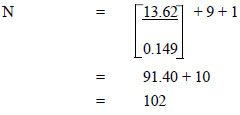 |
The desired sample size was decided to be at least 102 participants. The sample size assumed a 10% attrition rate [23]. The total number of participants in this study was 112.
The study recruited the medical chart of pregnant women who met the criteria for the study at the time after discharge from the hospital. The inclusion criteria were: 1) singleton, 2) gestational age between 37+0 and 41+6 weeks of pregnancy, 3) vertex position, 4) cervical dilate more than 4 cm. and effacement more than 80% in primiparous or cervical dilate more than 3 cm. and effacement more than 100% in multiparous, and 5) without having any complications to the mother and the fetus. The exclusion criteria were: 1) giving birth has undergone obstetric procedures including cesarean section, forceps extraction, and vacuum suction, 2) information in the medical record is incomplete, 3) the labouring woman received a painless labour procedure during childbirth.
During the study period, 4006 women gave birth at the Thammasat Hospital. 1963 had a normal delivery. The participants were selected via the stratified random-sampling technique. The researcher intended to collect a sample who met the inclusion criteria and then split them into two sub-groups by parity, nulliparous and primiparous. Then, systematic random sampling was used to recruit participants representing the population in each group. The recruited number of participants eligible for the study was 112 subjects. Four subjects were excluded from the study according to the outlier criteria. There were a total of 108 research participants remaining.
Research instruments included a questionnaire on the “socio-demographic,” “obstetrical,” and “clinical-feature” characteristics of the participants and “the progression of labour record”. The content validity was validated by five experts [24] comprising (a) three doctoral-level nurses who were structure specialists with at least 10 years of experience in the teaching profession and (b) two physicians with a minimum of 10 years of experience in obstetrics. The index of item-objective congruence (IOC) quantitatively evaluated the judgments of the content experts. A score = 1 indicated that the expert was personally certain that this item really measured the attribute. A score = -1 indicated that the expert was certain that this item did not measure the attribute. A score = 0 indicated that the expert was not certain whether the item measured or did not measure the expected attribute. Qualified items were expected to have an IOC equal to or greater than 0.50 [25]. In this study, the total IOC was 0.60. The reliability was tested prior to application in this study. Inter-rater reliability was used to assess the reliability of the instrument between the researcher and two nurses who work in labour unit with at least 5 years of experience. Total 30 chart data were selected to evaluate the reliability and record the error of progression of labour in the interater reliability record sheet. The result is 80%
Statistical analysis was performed using SPSS version 26. Data were analyzed using descriptive statistics, Pearson’s correlation coefficient, and multiple regression analysis with the Stepwise regression method. Significance was set at a p-value of ≤ 0.05.
3. RESULTS
According to the findings, the mean age of participants was 28.33 (S.D = 5.95). Most of the participants were Buddhists (89.81%). The majority of participants were divorced or not living with a spouse (58.30%) and did not specify their occupation (41.70%). The socio-demographics of the participants are shown in Table 1.
| Variables | Percent / mean + S.D. |
|---|---|
| Age (years) | - |
| 15-20 | 10 (9.26%) |
| 21-25 | 27 (25.00%) |
| 26-30 | 35 (32.40%) |
| 31-35 | 23 (21.30%) |
| > 35 | 13 (12.00%) |
| Min = 15; max = 45, x̅ = 28.33, S.D. = 5.95 | - |
| Religion | - |
| Buddhist | 97 (89.81%) |
| Christian | 1 (0.93%) |
| Muslim | 5 (4.63%) |
| Not specified | 5 (4.63%) |
| Marital status | - |
| Married and living with a spouse | 45 (41.70%) |
| Divorced/not living with a spous | 63 (58.30%) |
| Occupation | - |
| Housewife | -4.60% |
| Employee | 22 (20.40%) |
| Government officer | 5 (4.60%) |
| Government employee | 3 (2.80%) |
| Personal business | 4 (3.70%) |
| Private employee | 12 (11.10%) |
| Trade | 4 (3.70%) |
| Student | 7 (6.50%) |
| Laborer | 1 (0.90%) |
| Not specified | 45 (41.70%) |
Most of the participants had the first gravidity (38.89%) and the majority of participants had the first parity (45.40%). However, if we include the number of participants who have given birth more than 1 time, the total number will be 56.40 percent. The mean gestational age was 38.83 (S.D. = 0.85) weeks. The mean of height was 157.88 (S.D. = 0.57) cm. The mean of BMI was 28.71 (S.D. = 0.49). The mean of estimated fetal weight was 3,021.10 (S.D. = 29.04) gm. The mean duration of oxytocin exposure before delivery was 111.74 (S.D. = 12.31) min. The mean amount of oxytocin received before giving birth was 533.74 (S.D. = 63.54) mU.
Most of the participants had spontaneous rupture of membrane (60.20%), and received medication to induce labor (67.60%). The majority of participants had 4 cm of cervical dilatation (49.10%), cervix 100% effacement (62.96%), and the baby's head was one centimeter above the ischial spines (64.80%) at the first time of admission.
The mean length in the first stage, second stage, third stage and total time of delivery was 477.78 (S.D. = 20.39) min, 14.19 (S.D. = 9.89) min, 4.85 (S.D. = 3.26) min, and 496.53 (S.D. = 213.22) min, respectively. The obstetrical characteristics of the participants are shown in Table 2.
| Variables | Percent / mean + S.D. |
|---|---|
| Gravida | - |
| First | 42 (38.89%) |
| Second | 38 (35.19%) |
| Third | 20 (18.52%) |
| Fourth | 6 (5.56%) |
| Fifth | 2 (1.85%) |
| Parity | - |
| First | 49 (45.40%) |
| Second | 35 (32.40%) |
| Third | 19 (17.60%) |
| Fourth | 4 (3.70%) |
| Fifth | 1 (0.90%) |
| Gestational age (weeks) | - |
| 37 - 38 | 21 (19.44%) |
| 38+1 - 39 | 33 (30.56%) |
| 39+1 - 40 | 46 (42.59%) |
| 40+1 - 41 | 7 (6.48%) |
| 41+1 - 41+6 | 1 (0.93%) |
| Min = 37; max = 41+2, x̅ = 38.83, S.D. = 0.85 | - |
| Height (cm) | - |
| < 150 | 20 (18.52%) |
| 151-160 | 58 (53.70%) |
| 161-170 | 29 (26.85%) |
| > 170 | 1 (0.93%) |
| Min = 145 cm, max = 172 cm, x̅ = 157.88, S.D. = 0.57 | - |
| BMI | - |
| Underweight range (< 18.50) | 0 (0.00%) |
| Healthy weight range (18.50-24.99) | 14 (12.96%) |
| Overweight range (25.00-29.99) | 11 (10.19%) |
| Obesity class I (30.00-34.99) | 41 (37.96%) |
| Obesity class II (35.00-39.99) | 42 (38.89%) |
| Obesity class III (> 40.00) | 0 (0.00% |
| Min = 20.27, max = 43.00, x̅ = 28.71, S.D. = 0.49 | - |
| Rupture of membrane | - |
| Spontaneous | 65 (60.20%) |
| Artificial | 43 (39.80%) |
| Estimate fetal weight | - |
| SGA (<10th percentile) | 3 (2.78%) |
| AGA (10th-90th percentile) | 105 (97.22%) |
| LGA (> 90th percentile) | 0 (0.00%) |
| Min = 2,225 g, max = 3,863 g, x̅ = 3,021.10, S.D. = 29.04 | - |
| Receiving medication to induce labor | - |
| No | 35 (32.40%) |
| Yes | 73 (67.60%) |
| Amount of oxytocin received before giving birth (mU) | - |
| 0 -500 | 65 (60.19%) |
| 501-1,000 | 19 (17.59%) |
| 1,001-1,500 | 17 (15.74%) |
| 1,501-2,000 | 2 (1.85%) |
| 2,001-2,500 | 3 (2.78%) |
| 2,501-3,000 | 1 (0.93%) |
| 3,001-3,500 | 1 (0.93%) |
| Min = 0, max = 3,576, x̅ = 533.74, S.D. = 63.54 | - |
| The duration of oxytocin exposure before delivery (min) | - |
| 0 -60 | 49 (45.37%) |
| 61-120 | 20 (18.52%) |
| 121-180 | 12 (11.11%) |
| 181-240 | 10 (9.26%) |
| 241-300 | 6 (5.56%) |
| 301-360 | 3 (2.78%) |
| 361-420 | 6 (5.56%) |
| 421-480 | 1 (0.93%) |
| 481-540 | 0 (0.00%) |
| 541-600 | 0 (0.00%) |
| 601-660 | 1 (0.93%) |
| Min = 0, max = 616, x̅ = 111.74, S.D. = 12.31 | - |
| Length of first stage of labor (min) | - |
| 150-345 | 32 (29.63%) |
| 346-540 | 45 (41.67%) |
| 541-735 | 18 (16.67%) |
| 736-930 | 8 (7.41%) |
| 931-1,125 | 5 (4.63%) |
| Min = 150, max = 1,125, x̅ = 477.78, S.D. = 20.39 | - |
| Length of second stage of labor (min) | - |
| 1 - 11.00 | 45 (41.67%) |
| 11.01 - 20.20 | 41 (37.96%) |
| 20.21 - 30.20 | 12 (11.11%) |
| 30.21 - 39.40 | 6 (5.56%) |
| 39.41 - 49.00 | 4 (3.70%) |
| Min = 1, max = 49, x̅ = 14.19, S.D. = 9.89 | - |
| Length of third stage of labor (min) | - |
| 1 - 4.80 | 59 (54.63%) |
| 4.81 - 8.60 | 40 (37.04%) |
| 8.61 - 12.40 | 6 (5.56%) |
| 12.41 -16.20 | 1 (0.93%) |
| 16.21 - 20.00 | 2 (1.85%) |
| Min = 1, max = 20 min, x̅ = 4.85, S.D. = 3.26 | - |
| Total time of delivery (min) | - |
| 156 - 354.40 | 30 (27.78%) |
| 354.41 - 552.80 | 46 (42.59%) |
| 552.81 - 751.20 | 19 (17.59%) |
| 751.21 - 949.60 | 8 (7.41%) |
| 949.61 - 1,148.00 | 5 (4.63%) |
| Min = 156 min Max = 1,148 min, x̅ = 496.53, S.D. = 213.22 | - |
| Fetal body weight (g) | - |
| SGA < 2,500 | 5 (4.63%) |
| AGA = 2,501 - 3,999 | 102 (94.44%) |
| LGA) > 4,000 | 1(0.93%) |
| Min = 2,340, max = 4,000, x̅ = 3,101.51, S.D. = 348.05 | - |
| The cervical dilatation at time of admission (cm) | - |
| 3 | 10 (9.30%) |
| 4 | 53 (49.10%) |
| 5 | 26 (24.10%) |
| 6 | 9 (8.30%) |
| 7 | 7 (6.50%) |
| 8 | 2 (1.90%) |
| 9 | 1 (0.90%) |
| Cervical effacement | - |
| 75-80% | 40 (37.04%) |
| 100% | 68 (62.96%) |
| Station of fetal presentation | - |
| -2 | 17 (15.70%) |
| -1 | 70 (64.80%) |
| 0 | 21 (19.40%) |
| Variable | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | Y |
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | 1.000 | -.161 | .285 | -.021 | -.079 | -.062 | .165 | .136 | .183 | .134 |
| X2 | - | 1.000 | .053 | -.133 | -.129 | -.135 | .102 | .101 | -.174 | .009 |
| X3 | - | - | 1.000 | .069 | -.067 | .010 | .053 | .057 | .230 | .080 |
| X4 | - | - | - | 1.000 | .288 | .166 | -.111 | -.143 | -.087 | -.415* |
| X5 | - | - | - | - | 1.000 | .402 | .005 | -.004 | .033 | -.197 |
| X6 | - | - | - | - | - | 1.000 | -.169 | -.156 | .029 | -.142 |
| X7 | - | - | - | - | - | - | 1.000 | .972 | -.158 | .381 |
| X8 | - | - | - | - | - | - | - | 1.000 | -.168 | .435* |
| X9 | - | - | - | - | - | - | - | - | 1.000 | -.154** |
| Y | - | - | - | - | - | - | - | - | - | 1.000 |
Abbreviations:X1 = body mass index, X2 = height, X3 = estimate fetal weight, X4 = cervical dilatation, X5 = cervical effacement, X6 = the Station of fetal presentation, X7=dose of oxytocin exposure, X8 = the duration of oxytocin exposure, X9 = the parity, and Y = the duration of delivery.
| Variable | R | R2 | Adjust R2 | Std. Erroor of the Estimate | F |
|---|---|---|---|---|---|
| X8 | .435 | .189 | .181 | 93.576 | .189* |
| X8 X4 | .562 | .316 | .303 | 86.358 | .127* |
Abbreviations: X4 = cervical dilatation, X8 = the duration of oxytocin exposure.
From Table 3, there were 2 independent variables that had statistically significant differences at the 0.01 level: the duration of oxytocin exposure (X8) and the dilatation of the cervix (X4). One independent variable that had statistically significant differences at the 0.05 level was the parity (X9).
From Table 4, the results of the stepwise multiple regression analysis showed the factors that affect the duration of delivery in low-risk pregnant women. Out of a total of 9 independent variables, there are 2 independent variables that were statistically significant differences at the 0.01 level: the duration of oxytocin exposure (X8) and the dilatation of the cervix (X4), which can be explained as follows.
The duration of exposure to oxytocin (X8) has the greatest effect that can be predicted on the time to delivery with statistical significance at the 0.01 level, with a regression coefficient or predictive power of 0.181. It can explain that the duration of exposure to oxytocin will affect the duration of delivery by 18.10 percent while adding this variable separately.
When the factor of cervical dilatation (X4) was added, the regression coefficient or predictive power increased by 0.303, which increased when testing the difference with the original regression coefficient or predictive power. It was found that there was still statistical significance at the 0.01 level and it was found that the dilatation of the cervix increased affected the duration of delivery by 30.30 percent complied with the duration of exposure to oxytocin.
Six independent variables including body mass index (X1), height (X2), estimated fetal weight (X3), cervical effacement (X5), the Station of fetal presentation (X6), and dose of oxytocin exposure (X7) were removed from the equation because the difference was no statistically significant in this study. The parity (X9) has a statistically significant correlation (p < .05), however, after stepwise regression analysis, it has no power of prediction.
From Table 5, it is found that the duration of childbirth is influenced by the duration of oxytocin exposure (X8) and cervical dilatation (X4), respectively, which were statistically significant at the .01 level. The β value of the duration of exposure to oxytocin was 0.310, which means that all other factors are determined exactly the same in finding the length of time it takes to give birth. When the duration of exposure to oxytocin increased by 1 minute, it resulted in an increase in the duration of delivery by 0.310 minutes. While the β value of the cervical dilatation was -31.515 which means an increasing 1 centimetere of cervical dilatation results in a decrease in the duration time to delivery of 31.515 minutes. Therefore, the results of data analysis can be used to create a prediction equation in the form of raw scores as follows:
Equation
Y = a + β n (X8+ X4)
Y = 290.882 + 0.310 (the duration of oxytocin exposure) - 31.515 (cervical dilatation)
Coefficient of correlation (R) = 0.562
Coefficient of Determination (R Square) = 0.316
Adjusted R-squared = 0.303
Std.Error Square = 86.358
| Variable | β | Std.Error | Beta |
|---|---|---|---|
| X8 | 0.310 | 0.066 | 0.383 |
| X4 | -31.515 | 7.144 | -0.363 |
| a (Constant) | 290.882 | 35.878 | - |
R Square = 0.316 Std.Error Square = 86.358 Durbin-Watson =1.951.
It can be seen that when the two independent variables are brought into the forecast equation, the multiple correlation coefficient (R) = 0.562. The regression coefficient or adjusted predictive power (Adjusted R Square) = 0.316 or the prediction equation can predict the time of delivery at 31.60 percent.
4. DISCUSSION
This study aims to investigate the relationship between cervical dilatation, cervical effacement, the station of fetal presentation, body mass index, maternal height, estimated fetal weight, dose and duration of oxytocin exposure, and parity on duration of time to delivery in low-risk pregnant women. Our main finding is that the duration of oxytocin exposure, cervical dilation and parity were markedly affected in addition to the duration of time to delivery.
The duration of oxytocin exposure was positively related to the length of delivery (r = .43) and was a statistically significant predictor of the time to delivery (β = .383, p < 0.001). Our studies have shown that short-term exposure to oxytocin results in faster delivery. Oxytocin is a hormone that is important for uterine contraction by acting on the oxytocin receptor (OTR) [25]. A G-protein coupled receptor on the endometrium and myometrium of the uterus stimulates voltage-regulated calcium channels, which in turn causes calcium secretion (calcium influx), which enters the cell and causes the sarcoplasmic reticulum to release calcium, thereby intensifying the contraction of the uterus. Thus, pregnant women who received oxytocin had a good uterine contraction resulting in the short duration of delivery. While the dose of oxytocin exposure did not statistically affect the duration of delivery, in patients who had received oxytocin while waiting to give birth. G-protein on the surface of uterine muscle cells changed with a decrease in receptors (down-regulates oxytocin receptors) that decreased expression of messenger RNA (mRNA) and oxytocin binding sites, resulting in a reduction in the contractile response of uterine muscle cells [26, 27]. In vitro and clinical studies have shown that this reduction in response depends on both the dose and duration of exposure to oxytocin [28-30]. In our study, it was found that 84.26% of pregnant women received oxytocin between 1-4 hours before delivery, and 15.76% received the drug more than 5 hours. Robinson C. et al. (2003) reported that if oxytocin is administered for 4.2 hours, a response found OTR activity is reduced by up to 50% and does not respond at all if administered more than 6 hours [26]. This condition may persist for more than 90 minutes even after stopping oxytocin [31]. Thus, we can conclude that health professionals should also consider the appropriate time for giving oxytocin to the labouring woman during childbirth.
The cervical dilation was negatively related to the length of delivery (r = -.415) and had a statistically significant ability to predict time to delivery (β -0.363, p < 0.001). Studies have shown that women with greater cervical dilation have a shorter duration of labor. Cervical dilation is the process by which a woman gives birth, opening the cervix, the lowest part of the uterus. One indication that a woman is in labor is when her cervix opens up or dilates. It is generally safe to anticipate a consistent cervical dilation every hour once the active stage of labor begins. Many women don't begin to dilate more frequently until they are closer to 6 cm. The cervix opens during labor to allow the baby's head to pass through the vagina. When a woman's cervix is completely effaced (thinned out) and dilated to a length of 10 cm, the first stage of labor comes to an end. The pattern for delivering during the active phase is slightly different in primiparous and multiparous pregnancies, the average cervical dilatation rate is at least 1.2 cm per hour in primiparous pregnant women and 1.5 centimeters in multiparous pregnant women, respectively [32]. Our findings are similar to the study of Mariarosaria Di Tommaso and colleagues (2015), who found that women who had cervical dilatation between 0 and 2 cm had an average of 0-2.5 days for hospital admissions and duration before childbirth, while women who had cervical dilatation between 3 and 6 cm had an average of 0-1.3 days for hospital treatment and time before childbirth, with a statistically significant difference (p = .01) [33]. Similar to the study of Rosli et al. (2023), a study shows the effects of intrapartum care and labor outcomes in groups with cervical dilatation of 4 cm and 6 cm. The findings indicated that the 6 cm group had a significantly shorter mean time from the diagnosis of the active phase of labor until delivery (p < .001) [34].
Our finding found that the parity was negatively related to the length of delivery (r = -.154) but had no statistically significant ability to predict time to delivery. Higher parity, or the number of prior births, is known to be linked to faster labor [9, 35]. Shorter conditional times to the first stage of labor's completion during term pregnancy are linked to higher parities. It is reasonable to anticipate rapid labor for multiparous women experiencing spontaneous labor [21]. Compared to nulliparas (383 min for parity = 0), multiparas had a significantly faster labor progression from 4 to 10 cm (293, 300, and 313 min, respectively, for parity = 1, parity = 2, and parity = 3 +), as well as a shorter second stage of labor [8]. Vahratian A. et al.'s (2004) study did not find any statistically significant variations in the length of the active phase or the second stage of labor among multiparas. There seems to be no relationship between more childbearing and the evolution of labor among multiparous subgroups. In this study, there was a significant correlation between individual parity and labor duration (Pearson correlation = -0.027, p < .05). Interestingly, despite being shorter, the labor period did not differ statistically between multiparas and nulliparas. However, our results are in contrast with a study by Nesheim (1988) [9], who found no relationship between duration and parity greater than one.
There are six variables that were not statistically significantly affected by the duration of delivery, such as body mass index, height, estimated fetal weight, cervical effacement, the station of fetal presentation, and dose of oxytocin exposure that can be explained as follows:
A BMI of 30 kg/m2 or higher at the initial prenatal visit indicates obesity in pregnancy. A person's weight in kilograms divided by their height in meters squared yields their BMI, which is a straightforward weight-for-height index (kg/m2). Three distinct categories of obesity exist: class I, defined as BMI 30.0-34.9; class II, defined as BMI 35.0-39.9; and class III, or morbid obesity, defined as BMI 40 and above [36]. These classifications acknowledge the ongoing correlation between BMI and morbidity and mortality. The outcome of pregnancy is correlated with body mass index. Pregnancy complications that are more likely to occur when a woman has a high BMI include gestational diabetes. Pregnancy-related high body mass index (BMI) has been associated with an increased risk of a number of health issues for the unborn child, including fetal macrosomia, which causes the unborn child to be significantly larger than average at birth and increases the risk of complications from a C-section and prolonged labor. The obese group had higher rates of cesarean sections and instrumental births [37, 38]. Compared to women of normal weight, obese women had a higher likelihood of induced births and a longer first stage of labor [39]. Obese women may have uterine dystocia [40-42], which can cause cephalopelvic disproportion [43] and an increase in the time of dilation. According to the study of Carlhäll and colleagues (2013), the length of labor increased significantly as the mother's BMI increased. For obese women, the average length of labor increased dramatically. Comparing women with weight Normal (BMI 18.5-24.9) = 8.8h (p < .001) to women with Class I obesity (BMI 30-34.9), Class II obesity (BMI 35-39.9), and Class III obesity (BMI > 40) = 9.1 hours, 9.2 hours, and 9.8 hours, respectively [44]. However, our study discovered no connection between the timing of delivery and body mass index. This resemblance to the study of Ellekjaer and colleagues (2017) discovered that pregnant women with high BMIs did not have longer delivery times than women with normal BMIs. This is possible because the body mass index is not the only factor that affects the time of delivery [45].
The length of labor has been found to be inversely correlated with maternal height [46]; however, this correlation was not observed in our sample. Maternal height is often associated with their pelvic size. In obstetrics, the size of the pelvis is crucial. It's an excellent tool for predicting delivery methods. Several studies have identified a relationship between a woman's height and the shape of her pelvis and birth outcomes, including the incidence of fetal head and maternal pelvic space disproportionality in women who are shorter than 151 centimeters [47]. Mother's height is related to birth. Mothers with a height of 122-136 centimeters were associated with 43%, while mothers with a height of 137-157 centimeters had 35% chance of cesarean section [48]. However, in this study, the results showed that the mother's height did not affect the time it took to give birth. This is consistent with a study by Prasad & Taher (2002), which found that the mother's height was not related to the duration of delivery [49]. The results of our research can be discussed as follows, although the theory mentions factors affecting the delivery process, namely the 3Ps, force of birth (Power), route of delivery (Passage), and fetus (Passenger). The height of the mother is related to the passage of birth, which includes both the Bony passage and the soft passage. The Bony passage is the mother's pelvis. Because each woman's pelvis is a different size and shape, it affects vaginal birth and can be assessed. But it cannot make any changes and must be evaluated together with the size of the baby (Passenger) as well. Estimating fetal's weight is extremely important that used to plan care during pregnancy, during birth, and after birth correctly. The weight of the fetus is related to the duration of the birth. High birth weight results in a longer birth [50], and low birth weight results in a premature birth [51]. However, vaginal birth can occur when both the size of the mother's pelvis and the size of the baby are proportional. Because even though the mother has a relatively narrow pelvis if the baby is not very large, it may be possible to have a vaginal birth without any problems. In this study, the participants had a height of more than 150 cm and the infant’s body weight not exceeding 4,000 grams in the sample did not result in any difference in the time of delivery.
Fetal station, cervical dilation and cervical effacement are important to monitor during childbirth. It helps healthcare professionals evaluate how labor is progressing. Cervical effacement is the thinness of the uterus caused by the cervical muscles being pulled upwards and stretching of the lower uterine muscles, causing the cervix to become shorter and thinner, respectively, until the edge of the cervix cannot be felt. By comparing the length or thickness of the cervix, which is normally 2 centimeters long/thick, if it is shortened/thick 1 centimeter, the thinness is equal to 50 percent. If it is shortened/thick 0.5 centimeters, the thinness is equal to 75 percent, which in the cervix fully dilatation stage is usually the edge of the cervix cannot be felt. This is called 100% thinness. Thinness of the uterus occurs along with the opening and expansion of the cervix. As birth approaches, the cervix becomes thinner. The effacement and dilatation of the cervical work together. Fetal station refers to the depth at which the baby's head has lowered into the pelvis. A number between -5 and +5 indicates the location of the baby's presenting part, which is typically the head [52]. The measurement of fetal station, cervical dilation and cervical effacement can be done by pelvis examination and Ultrasound. However, health professionals at Thammasart University used mostly per vaginal examination, a subjective measurement that each health professional could determine with different values. There’s no precise way for health professionals to know exactly what exact value at any given moment—they can just estimate it by feel. Since there’s no definitive gauge, health professionals may measure differently.
CONCLUSION
The duration of childbirth varies from person to person. In this study, the duration of oxytocin exposure and the cervical dilatation are significant variables that most influence the time of delivery. Our finding shows that short-term oxytocin exposure leads to faster delivery. Giving medication to induce labor should take into account the duration of drug administration. The progress of labor should be closely monitored if the drug is administered for more than 4 hours. Another finding is women with higher cervical dilatation have shorter labor times. Thus, accurate assessment is therefore important for medical personnel to be aware of in caring for laboring women. It is recommended that vaginal examinations should be performed by experienced personnel, and a single person should be used to evaluate each laboring woman. There should be using precise instruments to assess the condition of the cervix, along with vaginal examinations by medical personnel.
These findings demonstrate the mathematical equation model for use in predicting the time to delivery. The statistical analysis used in this study is multiple linear regression, which creates an equation for predicting one dependent variable from several independent variables. Therefore, there is no examination of multiple causal variables to explain the dependent variable of interest. Further studies should be conducted on this issue. A higher labor prediction accuracy time could help with clinical management and help medical professionals get ready for an efficient delivery.
THE LIMITATION OF THE STUDY
The main limitation of this study lies in its retrospective design. Therefore, some data that affected the duration of labor, such as abdominal compression to help with birth, was not recorded. This may result in discrepancies in the study results. Another limitation was that a woman's dilation, effacement and descent of the presenting part are used to gauge how far along the labor is by periodically examining the vagina. Digital examination of the cervix (DEC) is standard. It is the accepted technique for assessing the status of delivery. One benefit of DEC is that it doesn't require specialized equipment and may be done at any time. But the disadvantage of DEC is subjective and there's a chance of inter-examiner error. More accurate measurements should be performed by the same provider. However, implemented by the same provider was not conducted for the majority of the women in our study.
Another thing is that the time when the cervix is completely open may not be the actual time but the time when it started to be detected. This causes the time period used to calculate in the equation to be inaccurate. Finally, the sample included in this study were only low-risk pregnant women from Asia, so the results may not generalize the findings of a study to other situations, people, settings, and measures.
AUTHORS’ CONTRIBUTION
The authors confirm their contribution to the paper as follows: study conception and design: KC; data collection: PS. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BMI | = Body Mass Index |
| OTR | = Oxytocin Receptor |
| DEC | = Digital Examination of the Cervix |
| IOC | = Item-objective Congruence |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Human Research Ethics Committee of Thammasat University, Thailand, No. 3, Project Number 66NU029 (COA. No. 039/2566).
HUMAN AND ANIMAL RIGHTS
All procedures performed in the study involving human participants were in accordance with the ethical standards of institution and /or research committee and with the 1975 Declaration of the Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
This study was a retrospective descriptive study. The study recruited the medical chart of pregnant women who met the criteria for the study at the time after discharge from the hospital. Therefore, the sample group was not asked to sign a consent form.
AVAILABILITY OF DATA AND MATERIAL
The data that support the findings of this study are available from the corresponding author on request [K.C-N.].


