All published articles of this journal are available on ScienceDirect.
Impact of a Neonatal Early-onset Sepsis Risk Calculator on Antibiotic Use in a Regional Australian Hospital
Abstract
Aims
The aim of this study is to evaluate the safety and efficacy of the online neonatal EOS Calculator at an Australian regional hospital. In addition, the utility of commonly used biomarkers as screening tools for suspected Early Onset Sepsis (EOS) was also assessed.
Background
Early onset sepsis is a potentially fatal condition; however, it is also rare, and remains a diagnostic challenge. Despite evidence against the use of non-specific infection biomarkers in neonates, many neonatal facilities worldwide continue to use these to investigate and guide management of neonatal EOS. Nevertheless, there is little research regarding use of the neonatal EOS Calculator in Australian and non-tertiary facilities.
Objective
This study sought to evaluate the safety and efficacy of the online neonatal EOS Calculator at an Australian regional hospital.
Methods
Retrospective review of neonates born at ≥34+0 weeks gestation investigated and/or treated for presumed early onset sepsis across a two-year period within a 224-bed regional acute hospital in Victoria, Australia. Actual management was compared to neonatal EOS Calculator recommendations to determine the potential reduction in investigations and empiric intravenous antibiotic use. Outcome data and blood culture results were used to assess safety. Levels of commonly used biomarkers were compared to EOS calculation and clinical examination findings.
Results
Retrospective application of the EOS Calculator among 296 subjects with presumed EOS was shown to reduce investigation by 44.3% and empirical antibiotic use by 48.9%. No true cases of culture-positive sepsis were identified. Elevated initial C-reactive protein (CRP) correlated positively with high EOS Calculation results and clinical illness on examination; however, there was absent or negative correlation of EOS risk with other biomarkers.
Conclusion
Use of the neonatal EOS Calculator may substantially reduce rates of investigation and empirical antibiotic use at regional facilities. However, more data is needed to establish the safety of the calculator. Biomarkers are of low value in clinical decision making with well infants and may hinder decision making when compared to the EOS Calculator and clinical examination.
1. INTRODUCTION
Early onset sepsis (EOS) is a potentially fatal condition [1, 2]. However, it is also rare, affecting between 0.3 and 0.8 neonates per 1000 births in developed countries [1-4]. EOS remains a diagnostic challenge [5-7]. Despite evidence against the use of non-specific infection biomarkers in neonates, worldwide many neonatal facilities, including tertiary NICUs, currently continue to use these to investigate and guide management of neonatal EOS [6-11]. Blood cultures, while offering excellent sensitivity and specificity when obtained correctly, yield results within 24-48 hours, necessitating empiric antibiotic use while awaiting definitive diagnosis [6, 10, 12]. The number of neonates receiving parenteral antibiotics greatly exceeds those with culture-positive sepsis [1, 2, 5]. Antibiotic use is associated with infant-parent separation, delayed initiation of breastfeeding and parental anxiety [1, 13, 14]. Early antibiotic use and its impact on infant microbiome is the topic of current research and has potential associations with asthma, obesity, and type 1 diabetes [15-18]. Reducing unnecessary antibiotic use in neonates is therefore a vital initiative.
The Neonatal EOS Calculator is an evidence-based algorithm which provides risk estimates based upon objective clinical data which are readily available in most circumstances [1, 2, 19]. Multiple international studies have reported safe reductions in antibiotic use between 42-59% when using the EOS Calculator, with the Calculator being endorsed by the American Academy of Paediatrics [2, 5, 14, 20-22]. Two recent meta-analyses found the Calculator was associated with significant reductions in antibiotic use with no evidence of inferiority regarding safety when compared to conventional approaches. A third meta-analysis raised safety concerns regarding missed or delayed treatment, especially in the setting of maternal chorioamnionitis [2, 20, 22].
Studies in the Australian context are limited and there is no regional data to date regarding the safety and efficacy of the neonatal EOS Calculator [1, 20, 23]. Variations in antenatal Group-B Streptococcus (GBS) screening protocols and intrapartum antibiotic guidelines limit the applicability of international studies in the Australian context, which necessitates the need for local evidence. A single prospective study in an Australian tertiary centre yielded similar results to those observed in American and European research [1]. Although similar studies are being undertaken at large metropolitan tertiary centres in Australia, we are unaware of any current research in regional Australia.
Grampians Health is a health service that encompasses a number of health services and encompasses the main campus (Grampians Health Ballarat) servicing the Ballarat region and numerous smaller health services across the Grampians region. The paediatric service at the Grampians Health Ballarat supports neonates born at ≥32 weeks gestation and has a Special Care Nursery of 12 bed capacity. During the study period, there were 2822 births at the Grampians Health Ballarat and GBS screening is standard antenatal care. The evaluation and management of early onset sepsis was derived from a non-standardised assessment of risk factors (generally requiring 2 or more risk factors, such as prematurity, CTG abnormalities, maternal temperature, GBS status, antenatal antibiotics, prolonged rupture of membranes) along with clinical condition of the baby and screening blood tests. Rigid guidelines were not routinely used during this period, as was the case throughout most equivalent institutions the investigators are aware of. The purpose of this study was to establish whether using the Neonatal EOS Calculator in this health service would lead to a reduction in neonatal antibiotic use whilst maintaining patient safety. Adding to the limited body of research in the application of the Calculator in Australia, specifically, the regional context, it was anticipated that our findings will support a prospective trial of the Calculator including local and wider policy changes in the management of neonatal EOS.
As a secondary outcome measure, was to establish the relationship, if any, of commonly used biochemical tests used in neonates with suspected EOS to determine their utility in management decisions. The sensitivity and specificity of biochemical tests for EOS, in particular C-reactive protein (CRP), varies widely between studies and data suggest they are of limited value in this context [6, 7, 11]. We hypothesise that our findings will align with previous data and support the health service to reduce its reliance upon biochemical markers as screening tools, while encouraging the transition to the EOS calculator as an objective assessment tool.
2. METHODS
2.1. Design and Setting
A retrospective chart review was conducted to compare actual investigation and treatment of suspected EOS in neonates born at ≥34 weeks gestation within the regional health service with recommended investigation and treatment based upon the EOS Calculator. This study was approved by the institutional Human Research Ethics Committee (#48734).
2.3. Subjects
The initial sample was established using admission data for the Special Care Nursey (SCN) at Grampians Health Ballarat. Subjects were selected based on one or more ICD-10-AM diagnostic codes associated with the birth admission and relevant to neonatal sepsis or antibiotic use associated with a SCN admission entry on BOSSnetTM, the local digital medical record (Table 1).
Subjects were included if they met the following criteria:
- Born at 34+0 weeks or greater gestational age;
- Investigations (Full Blood examination [FBE], CRP, Blood cultures) AND/OR antibiotics commenced within 72hrs of birth; and
- Indication for investigations OR antibiotic use recorded as presumed sepsis.
Subjects were excluded from the study if any of the following applied:
- Blood cultures obtained at greater than 72 hours of life;
- Antibiotics commenced at greater than 72 hours of life;
- Born external to Grampians Health Ballarat;
- Re-presentation to hospital post discharge;
- Antibiotics administered for indication other than presumed sepsis; or
- Insufficient available information to operate the EOS Calculator, determine the Clinical Examination Category, blood culture results, or inability to determine the outcome of the admission.
2.4. Procedures
Demographic, biochemical, and clinical data were collected from the subject’s electronic medical records. Medical records, including progress notes and observation charts, were used to determine the subject’s clinical presentation at the time of commencement of antibiotics in keeping with the EOS Calculator’s clinical presentation guidelines. Outcome data including blood culture results and discharge destination (Home, Tertiary Transfer or Deceased) were recorded. The EOS Calculator was then applied to each subject to compare current recommen- dations for investigation and management to the actual management that occurred.
Local incidence of EOS per 1000 live births was integrated into the EOS Calculator. In previous studies, this figure ranged between 0.3 and 1 per 1000 live term births [1-4]. At the time of this study, the local incidence within the health service was unknown. Given the absence of studies within the region, a conservative incidence of 1 in 1000 live birth was used, based on national EOS incidence rates and current incidence among similar regional health services. The conservative figure was also selected to minimise effect bias to avoid overestimating potential reductions in antibiotic use, while offering a safety margin. As no true positive blood cultures were identified during this study, we were unable to improve upon this figure during our investigation.
2.5. Statistical Considerations
Data were cleaned, checked, and analysed using Statistical Package for the Social Sciences (SPSS, Version 25.0). Descriptive and inferential statistics were used including statistical tests such as Pearson’s correlation (r), independent sample t-test, and one-way ANOVAs. Preliminary analyses were undertaken to ensure no violations of assumptions were present. The strength of Pearson’s correlation coefficient, a measure of the strength of the association between variables, is defined as a large (r=0.50-1.0), medium (r=0.30-0.49), or small (r=0.10-0.29) association between variables. Significance was determined at two-tailed p≤0.05.
3. RESULTS
A total of 2822 births were identified in the study period and 402 birth admissions were assigned a diagnostic code relevant to sepsis or parenteral antibiotic use. After exclusion criteria were applied, 296 were included in the final analysis. Demographic and clinical features of the cohort are outlined in Table 2. Of these, 292 received parenteral antibiotics (Fig. 1). Three neonates were investigated for presumed EOS, however, did not receive antibiotics. Of these, two did not have blood cultures obtained. Lastly, EOS was suspected in one neonate; however, investigations were not initiated due to parental choice. No neonates were excluded based on insufficient available data. Maternal temperature was not recorded for 21 mothers and a temperature of 37.0°C was used in Calculator analysis as in previous studies [23].
| Diagnostic Code | Description |
|---|---|
| P36.0 | Sepsis of new-born due to streptococcus, group B |
| P36.1 | Sepsis of new-born due to other and unspecified streptococci |
| P36.2 | Sepsis of new-born due to Staphylococcus aureus |
| P36.3 | New-born sepsis due to other / unspecified staphylococci |
| P36.8 | Other bacterial sepsis of new-born |
| P36.9 | Bacterial sepsis of new-born, unspecified |
| Z03.71 | Observation of new-born for suspected infectious condition |
| Characteristics of Included Neonates | |
|---|---|
| Gestational age, n (%) - 34+0-36+6 weeks - 37+0-40+6 weeks - ≥41 weeks |
99 (33.4) 180 (60.8) 17 (5.7) |
| Birth weight (grams) - Mean ± SD - Small for gestational age, n (%) |
3170±652.4 21 (7.1) |
| Risk Factor Presence | |
| Group B Streptococcus (GBS) status at birth, n (%) - Negative - Positive - Unknown |
127 (42.9) 46 (15.5) 123 (41.6) |
| Rupture of membranes (ROM) duration, n (%) - <4 hours - 4-12 hours - >12 hours |
135 (45.6) 79 (26.7) 82 (27.7) |
| Maternal fever n (%) - ≥38.0°C - ≥39.0°C - Unknown |
11 (3.7) 1 (0.3) 21 (7.1) |
| Biomarker results | |
| Laboratory results, mean (range), n - White cell count - Neutrophil count - C-Reactive protein, initial - C-Reactive protein, peak |
16.6 (4.8-38.0), 295 9.1 (1.4-27.0), 295 5.1 (0-159), 296 10.4 (0-171), 294 |
| Clinical examination findings | |
| Clinical presentation* n (%) - Well - Equivocal - Clinical Illness Persistent need for CPAP Intubated Seizure APGAR score @5 minutes <5 |
99 (36.2) 41 (14.2) 151 (49.3) 141 (93.2) 5 (3.4) 3 (2.1) 3 (2.1) |
| Antibiotic use | |
| Age at commencement (hours) n (%) - <2 hours - 2-4 hours - >4 hours |
112 (38.4) 50 (17.1) 130 (49.6) |
| Duration of antibiotics n (%) | |
| - ≤36 hours - >36 hours - Unknown |
150 (51.4) 138 (47.3) 4 (1.4) |
Blood culture volumes were not recorded; however, in keeping with available evidence, 0.5-1.0ml for all neonatal blood cultures is practiced at the institution. Five blood cultures returned positive results, all of which were deemed to be contaminants (Table 3). One blood culture result was unavailable. No positive blood cultures with pathogenic organisms were recorded. In cases where Cerebral Spinal Fluid (CSF) was obtained, 5 showed elevated CSF white cell count, but there were no positive CSF cultures. One case of viral meningitis was identified (Parechovirus). A total of 12.2% (36) of neonates required to transfer to tertiary centres, and there were no recorded deaths. Return correspondence was unavailable for 4 neonates transferred to tertiary centres. These neonates had an unknown total duration of antibiotics; however, no other critical data were missing from their local records. Maternal temperatures of ≥38.0°C were recorded for 11 cases, only one of which met the clinical criteria for chorioamnionitis.
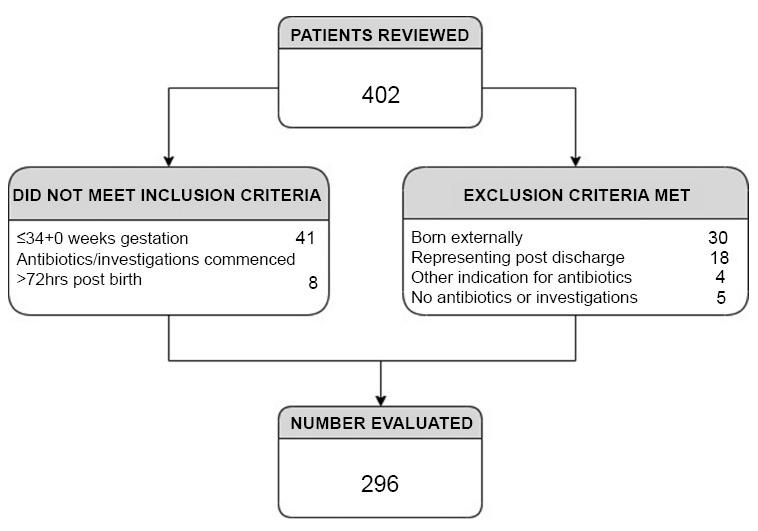
Population for analysis.
| Subject Number | Blood Culture Result |
|---|---|
| Patient 91 | Micrococcus spp. detected after 5 days of incubation. Probable skin contaminant. Repeat blood culture (obtained externally) negative. |
| Patient 170 | Coagulase negative staphylococcus detected in the paediatric bottle after 2 days incubation. Culture is of doubtful clinical significance. Probable skin contaminant. |
| Patient 278 | Micrococcus spp. detected after 2-days incubation. Culture of doubtful significance. |
| Patient 301 | Micrococcus spp. detected after 1-day incubation. Culture of doubtful significance. Repeat culture negative. |
| Patient 369 | Gram negative bacillus detected after 4 days incubation. 16S rRNA gene sequencing of this isolate gave close matches to Enhydrobacter aerosaccus and Moraxella osloensis but could not differentiate these species. Repeat culture negative. |
3.1. Primary Outcome Measure
On application of the Neonatal EOS Calculator to the cohort, 44.3% of neonates were recommended not to have blood cultures obtained and 49.0% were recommended not to receive empiric antibiotics.
3.2. Secondary Outcome Measures
Serum levels of CRP, both the initial and peak, total white cell count (WCC) and neutrophil count (where available) were compared to both clinical examination findings and EOS Calculator risk estimate. WCC and neutrophil counts were available for all subjects that were included, and CRP was available for all but one subject.
When examining the whole cohort for the association between biomarkers and clinical presentation, no biomarkers correlated with clinical examination findings, except for Neutrophil levels, which had a small negative correlation (r = -0.163, p < 0.005). When examining EOS calculator risk estimate and biomarker levels, CRP (initial), CRP (peak), and Neutrophil levels also had small negative correlations (Table 4).
|
Biochemical Markers |
Correlation (r) Significance (p) Number (n) |
Clinical Examination Findings |
Risk Estimate from EOS Calculator |
|---|---|---|---|
| CRP Initial | r | -0.095 | -.175 |
| p | 0.104 | 0.004** | |
| n | 296 | 274 | |
| CRP (peak) | r | -0.020 | -.156 |
| p | 0.727 | 0.010** | |
| n | 294 | 272 | |
| WCC | r | -0.070 | -0.083 |
| p | 0.232 | 0.172 | |
| n | 295 | 274 | |
| Neutrophils | r | -.163 | -.199 |
| p | 0.005** | 0.001** | |
| n | 295 | 274 |
|
Biochemical Markers |
Correlation (r) Significance (p) Number (n) |
Clinical Illness Group | High Risk Estimate group |
|---|---|---|---|
| CRP Initial | r | .249** | .234* |
| p | 0.003 | 0.020 | |
| n | 140 | 98 | |
| CRP (peak) | r | 0.163 | 0.180 |
| p | 0.056 | 0.078 | |
| n | 138 | 97 | |
| WCC | r | -0.120 | -0.152 |
| p | 0.158 | 0.135 | |
| n | 140 | 98 | |
| Neutrophils | r | -.170* | -.210* |
| p | 0.044 | 0.038 | |
| n | 140 | 98 |
Further, when investigating individual clinical examination groups (Well, Equivocal, Clinical illness), initial CRP correlated positively, while neutrophil levels correlated negatively among those who were classified as having a “clinical illness” on examination respectively (r = 0.249, p < 0.003; r = -0. 174, p < 0.044). No other groups (Well or equivocal) had any correlations between biomarkers. Similarly, initial CRP correlated positively, while neutrophil levels correlated negatively among those who were classified in the “High Risk Estimation EOS Calculation”. No other groups (Low or Moderate Risk Estimate EOS Calculation) had any correlations between biomarkers (Table 5).
4. DISCUSSION
The findings of the retrospective review support the EOS calculator as an effective measure to reduce empiric antibiotic use in the neonatal population within the regional health service. These findings, coupled with previous studies, justify a prospective study to be conducted within the health facility’s neonatal unit to examine the safety and efficacy of the Calculator. However, due to an absence of blood cultures and proven EOS cases, we cannot comment on the safety of the calculator in this study.
It is unfortunate, though not unexpected, that no positive blood cultures with pathogenic organisms were recorded. During the study period, 2822 births were recorded at the health service. The absence of positive blood cultures may reflect a local incidence of 0.3 per 1000 live births, a figure that is consistent with previous Australian studies. Blood culture volumes were not recorded, although staff are instructed to collect 0.5-1mL in keeping with current evidence.
The absence of local incidence data presents a challenge when investigating a rare condition, a difficulty amplified in a regional setting with smaller birthing numbers. Added to this, high-risk deliveries are transferred to larger tertiary facilities for birthing, while unwell neonates transferred to larger tertiary facilities may reduce the overall number of blood cultures obtained. We postulate that considering the birth numbers, retrospective data from the previous 10 years will be required to establish the local incidence of EOS. Though sought, this data was unavailable at the time of publication. Determining the incidence figure to use presents an ongoing difficulty for regional and rural health services that use the Neonatal EOS Calculator. Future research could address this further.
The findings related to the secondary outcome support the hypothesis that biochemical markers (initial and peak CRP, WCC and neutrophil count) correlate poorly with risk estimates from the EOS calculator. In this case, they are of limited utility in the decision to commence antibiotics. We conclude that CRP is not useful as a screening test in infants with low-risk EOS Calculations. A high CRP may influence a clinician to commence IV antibiotics, despite EOS calculator recommendations and an examination indicating a well-child. This would negatively impact the efforts to reduce antibiotic use.
While elevated initial CRP does positively correlate with a high-risk EOS Calculation, these subjects would receive empiric antibiotics regardless of CRP value should the EOS Calculator be introduced. Furthermore, with the introduction of the EOS Calculator, greater numbers of neonates at high risk of EOS may be identified and treated early, avoiding potentially missed cases when CRP has not been obtained. It will also omit treatment delays while awaiting CRP results or false negatives from non-elevated initial CRP. These findings support the decision to move away from using biochemical markers as decision aids in screening for EOS and utilising serial examination to determine clinical status.
Given the ubiquity of the use of CRP and WCC in evaluating infants with suspected sepsis at our facility, this may represent a cultural challenge during the implementation phase, thus careful education of clinicians will be essential. The authors recognise that many neonatal clinicians continue to utilise CRP in other clinical scenarios, for example, to guide the duration of treatment in infants commenced on IV antibiotics. The utility of CRP in this setting was not evaluated during this study and we cannot comment on this. Further studies may be warranted.
New methods to reduce antibiotic exposure among neonates are continuing to evolve – serial physical examination is emerging as a feasible and safe approach [24]. In considering future directions, it is important to consider the context of practice. At Grampians Health Ballarat, as in many regional Australian health services, the paediatric junior medical staff are rotating staff from other health services who have varied paediatric exposure and typically have limited neonatal experience. In these settings, it is valuable to have an objective assessment tool that is clearly understood by senior and junior staff alike, as afforded by the neonatal EOS Calculator.
4.1. Limitations
Study limitations include using retrospective rather than prospective data, along with a lack of blood culture volumes being recorded. The absence of local incidence data also presents a challenge and may reflect the smaller numbers of live births recorded in regional settings, suggesting prospective research across a larger catchment may be needed. Further, data may be limited due to high-risk deliveries and unwell neonates having the propensity to be transferred to larger tertiary facilities.
CONCLUSION
The EOS Calculator may effectively reduce empiric antibiotic use in our regional health care facility. Future directions, including a prospective trial require careful consideration of safety and clinician discretion based upon the clinical scenario. The secondary outcome findings support reducing reliance on biochemical markers such as CRP and WCC as screening tools in neonates suspected of EOS, and instead utilising the EOS Calculator and serial clinical evaluation.
AUTHOR CONTRIBUTIONS
Study conception and design: KZ, LD, and DT2; data collection: KZ; analysis and interpretation of results: KZ, LD, DT1, and DT2; draft manuscript: KZ, LD, BP, DT1, and DT2. All authors reviewed the results and approved the final version of the manuscript under the heading of Author Contribution.
LIST OF ABBREVIATIONS
| SCN | = Special Care Nursey |
| EOS | = Early Onset Sepsis |
| WCC | = White Cell Count |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ballarat Health Service Ethics Committee Australia (#48734).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIAL
The data presented in this study are available on request from the corresponding author [D.T].


