All published articles of this journal are available on ScienceDirect.
Educational Intervention on Cleaning and Sanitation of Surfaces in a Pediatric Hospitalization Unit
Abstract
Introduction:
Environmental surfaces may serve as a reservoir for various microorganisms and consequently, they represent a potential risk for the spread of healthcare-associated infections.
Objective:
This study aimed to assess the cleaning and sanitation of surfaces (CSS) before and after implementing a Standardization Program for Cleaning and Sanitation of Surfaces (SPCSS).
Methods:
An analytical, comparative, and intervention study was conducted from 2020 to 2021 in a pediatric hospitalization unit in Midwest Brazil. Four frequently touched surfaces were monitored before and after the cleaning and sanitation process using the following methods: Adenosine Triphosphate (ATP) quantification, Colony-Forming Unit (CFU) count, and visual inspection. The study consisted of three stages: stage I (situational diagnosis of the CSS process), stage II (implementation of the SPCSS), and stage III (assessment 60 days after implementing the program). A total of 576 assessments were performed in all three study stages.
Results:
The CSS process was effective in all three study stages by using the ATP and CFU methods. In stage I, statistically significant results were obtained for four surfaces using the ATP method, and two by the CFU count. In stages II and III, all surfaces presented lower ATP and CFU results (p<0.05). In the visual inspection, only the bathroom door handle (stage I: p=0.041; stage III: p=0.007) and toilet flush handle (stage I: p=0.026; stage III: p=0.007) passed the test.
Implications for Nursing:
This study presents subsidies to evaluate the cleaning and disinfection process carried out by the nursing and hygiene team.
Conclusion:
The SPCSS exerted a positive impact on the CSS process.
1. INTRODUCTION
Healthcare-Associated Infections (HAIs) are responsible for generating complications in the patients, such as prolonged hospitalizations, increased hospitalization costs, longer treatments, physical changes, and death during health services; thus, they have become a global public health challenge [1-3]. As they can house various microorganisms, surfaces represent a potential risk for the onset of HAIs. Factors related to the survival time of microorganisms include environmental condi-tions, such as temperature, wind, and humidity, and other as-pects concerning the type of surface and microbial load [4, 5].
In the hospital setting, environmental surfaces have become important routes of infection transmission, as they serve as reservoirs for microorganisms and allow for their dissemination. Contaminated surfaces, mainly those that are frequently touched, contribute to the transmission of pathogens. Therefore, hygiene measures in healthcare environments require a comprehensive approach in which different strategies may be implemented together, simultaneously to targeted and risk-based approaches, in order to reduce the risk of HAIs for the patients [6, 7].
It is worth considering that cleaning and sanitation of surfaces are often inadequate, as evidenced by studies that also show the importance of assessing the efficacy of these procedures and investing in the training of healthcare and cleaning professionals [8-10].
Added to the above is the fact that, with the COVID-19 pandemic period, the importance of environmental cleaning control has become even more evident to implement preventive measures [11]. A relevant way to prevent environmental contamination is frequent cleaning and sanitation of surfaces (CSS) [4]. However, without proper monitoring, the performance of this process does not ensure its purpose or effectiveness. Thus, it is fundamental to use methods to assess CSS efficiency. Among the methods and procedures available for monitoring the CSS process in health services, there are visual inspection, Adenosine Triphosphate (ATP) assessment and quantification, and Colony-Forming Unit (CFU) count [12]. Each method has specific characteristics and indications in relation to the monitoring of compliance and feedback [13]. The literature researched revealed the absence of educational intervention studies and standardization of procedures related to the CSS process involving professionals from the hygiene and cleaning teams of pediatric hospitalization units. Moreover, no methods were used to monitor this process in this specialty.
It is pertinent to highlight that children can acquire infections from various types of places; however, the transmission risk is greater in the hospitalization environment, as exposure is higher, and the patients are in critical health conditions and undergo invasive procedures. Children are at an increased risk for developing diseases, mainly those who lack immunity to infectious agents and those who are weakened or immunocompromised [14].
In the literature, it is possible to find several studies regarding the monitoring of the cleaning and disinfection process in a hospital context, mainly in intensive care units and surgical centers, but studies carried out in pediatric hospitalization units are limited. It is also justified the importance not only of implementing but also of monitoring standardization programs in health services, aiming at improving the safety and quality of care.
Therefore, the objective of this study was to assess CSS before and after implementing a Standardization Program for Cleaning and Sanitation of Surfaces (SPCSS).
2. MATERIALS AND METHODS
2.1. Study Design, Locus, and Period
An analytical, comparative, and intervention study was carried out. The research was conducted at a pediatric hospitalization unit of a hospital institution in the inland state of Mato Grosso do Sul, Brazil. The institution is a reference for 10 municipalities, namely: Água Clara, Aparecida do Taboado, Bataguassu, Brasilândia, Cassilândia, Inocência, Paranaíba, Santa Rita do Pardo, Selvíria and Três Lagoas, providing support to an approximate population of 255,000 inhabitants [15]. The hospital has a total of 185 beds, of which 20 are for pediatric patients [16]. The study was conducted from December, 2020 to March, 2021, during the COVID-19 pandemic.
2.2. Standard Protocol of the Institution
Considering all the work shifts (morning, afternoon, and night), the pediatric hospitalization unit has the following professionals in its team: 03 nurses, 06 nursing technicians, and 03 hygiene and cleaning team professionals (HCTPs), which are in charge of the CSS process in the unit, according to the institutional protocol. The estimated time for surface cleaning, including terminal and concurrent cleaning, was found to vary between 40 and 60 minutes. The cleaning routine was performed once a day in the morning in the entire unit and whenever requested, considering the presence of dirt. The nursing team was only in charge of sanitizing patient bed rails in concurrent cleaning, whereas HCTPs were responsible for cleaning the other surfaces.
The institution has a single Standard Operating Procedure (SOP) for all hospitalization units in order to standardize the actions of the hospital sanitation service. The last review/approval of the institution's CSS SOPs dates from May 3rd, 2019.
According to the protocol described in the SOP, surfaces are cleaned with soap and water and using a cotton cloth moistened with intermediate-level disinfectant and Peroxy 4D detergent composed of hydrogen peroxide 4.25%, coco dimethyl benzyl ammonium chloride, and didecyl dimethyl ammonium chloride 5.6%, which cleans and sanitizes in a single step (Spartan do Brasil, Produtos Químicos LTDA).
2.3. Study Protocol
The definition of surfaces to be assessed was based on the ones with a high frequency of contact, as they are strongly related to the dissemination of pathogens and thus, to the occurrence of HAIs [17, 18]. Based on this premise, the following surfaces were selected: the armchair, patient’s bed rail, inside door handle of patient's bathroom, and patient's toilet flush handle [19, 20].
Monitoring of the cleaning and sanitation process was done by a single researcher [21]. The surfaces were evaluated using three monitoring methods: CFU count, ATP quantification, and visual inspection. ATP quantification was performed through the bioluminescence technique using a cotton swab (3M™ Clean-Trace™ ATP Surface Test Swab), and the reading was expressed in Light Relative Units (LRUs) using a luminometer (Clean-Trace™ ATP System, 3M Company, St. Paul, MN), and the surfaces were approved when they obtained values below 250 LRUs. The total aerobic microbial count was performed using 24-cm RODAC® (Replicate Organism Direct Agar Contact) contact plates containing a Tryptic Soy Agar (TSA) combination with casein and soy peptones. TSA has Tween 80 and lecithin in its composition, chemical substances that inactivate phenolic and quaternary ammonium disinfectants (Plastlabor, Rio de Janeiro, Brazil). Surfaces were considered approved with values below 2.5 CFU/cm2 [22-26]. In relation to the visual inspection, the surfaces were considered approved if they did not present dust, stains, bodily fluids, fingerprints, and/or structural defects [27].
The surfaces were monitored according to the CSS routine of the sector before and after the HCTPs performed the CSS process, which was from 6 a.m. to 11 a.m. The samples were collected twice a week for four weeks per stage. Eight samples were collected both before and after the CSS process, totaling 192 assessments in each study phase Chart 1 [19, 20].
| Method |
Stage I (4 Collection Weeks) |
Stage II (4 Collection Weeks) |
Stage III (4 Collection Weeks) |
Total Assessments |
|---|---|---|---|---|
| Visual | 64 | 64 | 64 | 192 |
| ATP | 64 | 64 | 64 | 192 |
| CFU | 64 | 64 | 64 | 192 |
| TOTAL | 192 | 192 | 192 | 576 |
It is noted that the rooms selected for assessment of the CSS process were chosen by convenience, that is, according to the routine of the professionals who performed this process.
2.4. Study Phases
The study was conducted in three stages: 1. Situational diagnosis of the CSS process, 2. Implementation of the Standardization Program for Cleaning and Sanitation of Surfaces (SPCSS), and 3. Long-term assessment after implementing the program [20, 28-31].
The practices employed by the team to perform CSS were observed in stage I, such as the use of protocols, products, frequency, time, and friction. The surfaces were monitored using all three aforementioned methods. During this stage, the professionals in charge of CSS were not informed about the actual objective of the study in order to avoid the Hawthorne effect, preventing professionals from modifying their practices due to being observed [8, 32, 33].
An SPCSS was developed considering the data obtained in stage I. In this phase, the participants of the program were advised about the actual objective of the study and asked to sign a free and informed consent form. Implementation of the SPCSS consisted of three moments: dialogue lecture on environmental contamination and prevention strategies, demonstration and feedback of the team with the results of the monitoring obtained in stage I, and standardization of the practices with a protocol update, including the definition of cleaning frequency, friction, use of microfiber cloths (80% viscose, 15% polypropylene, and 5% polyester), and the proper way to moisten the cloth with the disinfectant already standardized in the institution. Implementation of the SPCSS lasted for approximately two hours, which were divided into 15-minute presentations and demonstrations, according to the workers' availability, in order not to disrupt the care routine of the professionals working in the sector [20, 28-31]. All workers in charge of CSS were invited; the participants were 02 nurses, 04 nursing technicians, and 02 HCTPs, all with 66.6% participation per category. This adherence percentage was due to the leaves, vacations, and distance from work during the team training period.
After implementing the SPCSS, CSS monitoring was performed again (Stage II), as done in stage I, for 30 days in order to verify if the SPCSS had any short-term effects [20, 28-31]. In this stage, feedback on the results was given to the professionals in charge of CSS.
Sixty days after stage II was concluded, CSS was monitored again (Stage III), using the same methods, in order to verify if the SPCSS had any long-term effects. Feedback was given to the team in this stage [20, 28-31].
2.5. Statistical Analysis
In this study, the statistical analysis included the Wilcoxon test to compare the results between the ATP measurement and the CFU counts. The Mann-Whitney test was chosen in order to compare CFU counts to ATP measurements. Both tests considered a 5% significance value (p<0.05).
For the quantitative approach, CFU and ATP data measurements were also performed to compare the study stages. In this sense, the quantitative data on total aerobic microbial count (CFU/cm2) and ATP were compared, and variation of these data was calculated through the following expression:
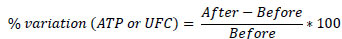
In the assessment of the surfaces through visual inspection in all the stages, Fisher's exact test for two proportions was applied to observe differences.
2.6. Ethical Aspects
The research followed all the national and international standards in relation to the ethical precepts for research studies involving human beings. The study was approved by the Committee of Ethics and Research with Human Beings of the Federal University of Mato Grosso do Sul, Brazil (CAAE: 29350219.8.0000.0021).
3. RESULTS
Based on the monitoring of all four surfaces by the three methods (ATP, CFU, and visual inspection), 192 assessments were conducted in each stage, totaling 576 evaluations in all three study stages.
Table 1 shows the results of the comparison between the situations before and after the cleaning and sanitation process corresponding to the four surfaces evaluated in all three study stages.
|
Analysis Method and Stages |
Cleaning |
Bathroom Door Handle |
p-value |
Toilet Flush Handle |
p-value | Bed Rail | p-value | Companion's Armchair | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Stage I | |||||||||
| ATP (LRU)1 | Before | 781 (72;5,065) | 0.010 | 436 (117;751) | 0.007 | 377 (46;3,807) | 0.010 | 551 (123;1,672) | 0.010 |
| After | 90 (29;365) | 65 (22;470) | 164 (38;562) | 158 (106;259) | |||||
| Bacteria (CFU/cm2)1 | Before | 75.5 (1;112) | 0.021 | 95 (25;110) | 0.007 | 69.5 (8;110) | 0.092 | 93.5 (12;108) | 0.054 |
| After | 16.5 (0;80) | 8.5 (2;71) | 15 (1;109) | 21 (6;115) | |||||
| Analysis of the variation2 | LRU | -86 (-98;9) | 0.494 | -64 (-95;-17) | 0.494 | -66 (-89;2) | 0.636 | -69 (-86;8) | 0.874 |
| CFU | -72 (-100;300) | -89 (-93;-26) | -82 (-99;1263) | -58 (-94;541) | |||||
| Stage II | |||||||||
| ATP (LRU)1 | Before | 63 (13;246) | 0.007 | 159 (29;400) | 0.007 | 269 (88;397) | 0.007 | 535 (286;868) | 0.007 |
| After | 24.5 (10;91) | 23.5 (8;50) | 15.5 (5;123) | 43.5 (7;145) | |||||
| Bacteria (CFU/cm2)1 | Before | 64.5 (9;102) | 0.007 | 89 (6;115) | 0.007 | 91 (24;110) | 0.007 | 81 (40;113) | 0.007 |
| After | 14 (7;64) | 14 (1;20) | 16.5 (3;65) | 26 (6;92) | |||||
| Analysis of the variation2 | LRU | -57.5 (-77;-18) | 0.636 | -69 (-90;-51) | 0.189 | -84 (-94;-34) | 0.636 | -67 (-92;-44) | 0.636 |
| CFU | -56 (-84;-6.3) | -83 (-96;-77) | -82 (-96;-27) | -49 (-94;-4.9) | |||||
| Stage III | |||||||||
| ATP (LRU)1 | Before | 105.5 (55;888) | 0.007 | 101.5 (33;455) | 0.007 | 130 (66;887) | 0.007 | 323 (221;993) | 0.007 |
| After | 46.5 (31;222) | 27 (11;131) | 55 (32;364) | 140 (92;644) | |||||
| Bacteria (CFU/cm2)1 | Before | 46.5 (21;110) | 0.007 | 46 (14;110) | 0.007 | 88 (10;108) | 0.007 | 93 (49;109) | 0.011 |
| After | 18 (10;99) | 10 (3;63) | 12 (3;55) | 28 (4;92) | |||||
| Analysis of the variation2 | LRU | -53 (-75;-29) | 0.874 | -68 (-88;-24) | 0.958 | -59 (-87;-35) | 0.318 | -52 (-70;-35) | 0.372 |
| CFU | -55 (-80;-10) | -62 (-94;-14) | -69 (-91;-10) | -64 (-93;0) | |||||
1p-value referring to the Wilcoxon test at p<0.05.
2p-value referring to the Mann-Whitney test at p<0.05. Values in bold type present significant differences at p<0.05.
Stage I showed significant differences between the LRU scores in the four surfaces assessed: bathroom door handle (p=0.010), toilet flush handle (p=0.007), bed rail (p=0.010), and companion's armchair (p=0.010). In all the cases, it was possible to assume that ATP quantification was significantly lower in the post-CSS stage.
With regard to microbial count, only two surfaces presented significant differences when comparing the CFU values, namely bathroom door handle (p=0.021) and toilet flush handle (p=0.007). In these surfaces, the post-CSS medians were significantly lower than the pre-CSS medians, evidencing that the process was effective.
Considering the ATP (LRU) and CFU variation analysis of stage 1, it was not possible to observe significant differences in the comparison of the surfaces evaluated.
Stage II showed significant differences for all surfaces analyzed with regard to ATP and CFU count, revealing that CSS was effective in reducing LRU and microbial load.
Considering the ATP (LRU) and CFU variation analysis in stage II, no significant differences were reported in the comparison of the surfaces evaluated.
The results of stage III were similar to those obtained in stage II. The four surfaces analyzed presented significant differences in the comparison of the results for ATP and CFU before and after CSS, and the process was considered effective by the monitoring methods.
The ATP (LRU) and CFU variation analysis of stage III did not evidence significant differences in the comparison of the surfaces evaluated either.
With regard to the results of proportions of the approved surfaces evaluated according to visual inspection, it is noted that, in stage I, there were differences (Fisher's exact test) in the proportions of two surfaces assessed by the visual test: bathroom door handle (p=0.041) and toilet flush handle (p=0.026). In both surfaces, the approval rates increased significantly from pre- to post-CSS: from 12.5% to 75% for the bathroom door handle and from 0% to 62.5% for the toilet flush handle.
For stage II, there were no significant differences in the proportions of approved surfaces in the pre-/post-CSS comparison. Stage III showed significant results for two surfaces: bathroom door handle (p=0.007) and toilet flush handle (p=0.007), with approval rates increasing from 25% to 100% for the first and from 0% to 75% for the second.
Fig. (1) shows the graph with the individual values corresponding to the ATP indices of all four surfaces in the three stages after the intervention. Values below 250 LRUs were considered as an indication that the surface had passed the test.
In stage I, no surface reached approval rates of 100%, and the approval rate was 75% for two surfaces (toilet flush handle and bed rail) and 87.5% for another two surfaces (bathroom door handle and companion's armchair). The approval percentages increased in stage II, with all surfaces presenting 100% for ATP, except for the companion's armchair (62.5%). In stage III, the approval rates were 100% for two surfaces (toilet flush handle and bathroom door handle), 87.5% for one surface (bed rail), and 75% for another one (companion's armchair). In general, the ATP results demonstrated that the companion's armchair was the surface with the lowest approval rates, regardless of the stage analyzed.
Microbial quantification (CFU/cm2) was assessed according to the 2.5 CFU/cm2 cutoff point and is presented in Fig. (2).
Most of the surfaces evaluated did not pass the test according to the approval criterion adopted by the microbial quantification method. Only three surfaces passed the test, namely bed rail, bathroom door handle, and toilet flush handle (in Stage I and Stage II). Despite the low percentage (25%), stage 1 was the one that presented the highest number of approved surfaces. These data suggest that the 2.5 CFU/cm2 cutoff point, which was adopted to consider that a surface was appropriately disinfected, was more difficult to reach when compared to the total aerobic count before and after the CSS process.
4. DISCUSSION
This study presents the results of the CSS monitoring performed in a pediatric hospitalization unit during the pandemic period. The SPCSS led to a positive result, mainly considering the percentages of approved surfaces after CSS by the team and the medians, based on CFU count and ATP measurements in most of the surfaces. Thus, in general, the measures implemented with the educational intervention led to a sustained improvement in the CSS process for all surfaces.
In this perspective, various studies [6] have shown a reduction in the rates of infection/colonization by epidemiologically important microorganisms, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococcus (VRE), and Clostridioides difficile, adopting measures such as improvement in the CSS process, consisting of professionals training in charge of cleaning, technique, product, audit and communication, a fact that not only improved the team's performance, knowledge and attitude, but also made it possible to reduce the occurrence of pathogenic microorganisms.
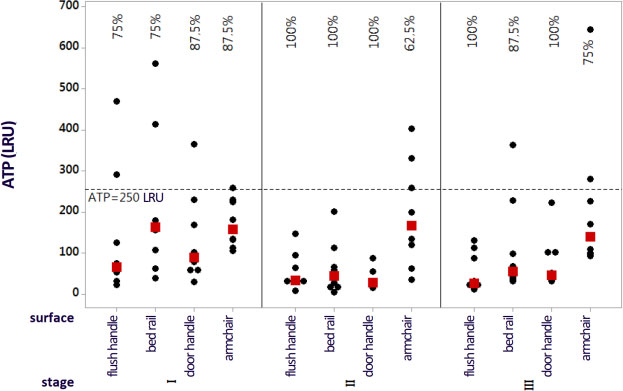
Note: Percentage values referring to the approval indices. The black dots indicate the individual ATP values, and the red dots represent the data medians.

Note: Percentage values referring to the approval indices. The black dots indicate the individual CFU/cm2 values, and the red dots represent the data medians.
In stage I, all the surfaces evaluated presented statistically significant reductions for ATP, and two of the surfaces for CFU. However, in stages II and III, after implementing the SPCSS, all surfaces had statistically significant reductions both for ATP and CFU, thus evidencing the efficiency of CSS. With regard to the visual inspection, it was also possible to observe an improvement in the pre-/post-CSS comparison for all the surfaces in all stages, except for the bed rail in stages II and III, which did not present any improvement.
This reduction in CFUs for all the surfaces evaluated, mainly in stages II and III, could certainly be attributed to the SPCSS since, among other reasons, it enabled the professionals to acquire diverse knowledge on environmental contamination and prevention strategies, as well as demonstration and feedback to the team, with the results of the monitoring obtained in stages I and II, as well as standardization of the practices with a protocol update.
The literature evidences a variation in relation to the effects of educational interventions, according to the study locus and the surfaces to be monitored. A study conducted in isolation rooms of a reference pediatric hospital in Cape City, South Africa, in order to assess pre- and post-terminal cleaning and its adequacy based on individual verbal feedback to the environmental cleaning workers, reported a significant reduction in the mean values for microbial count (p<0.001) and ATP (LRU) detection (p<0.001), as well as an improvement in fluorescent marker removal (p<0.001) between the initial and subsequent cleaning instances. However, surfaces cleaned less frequently in the first cleaning instance, such as toilet seats, toilet flush handles, paper towel dispensers, and door handles, did not present any improvement in fluorescent marker removal [34].
A research study conducted in a clinical and surgical unit reported that of the five surfaces evaluated, bathroom door handle (p=0.007) and toilet bowl (p=0.010) presented statistically significant differences for ATP in the pre-/post-CSS comparison. When assessing microbial count, only the toilet flush handle presented a statistically significant result (p=0.040) [20]. However, both the study conducted in a clinical and surgical unit and the current research, conducted in a pediatric unit, found a reduction in microbial load and the presence of organic matter by means of ATP measurement.
With regard to the educational intervention, a research study conducted in an Emergency Care Unit (24-h ECU) verified that the interventions improved the effectiveness of cleaning immediately, although this effect did not last in the long term [32]. This result differed from the data obtained in the pediatric unit, especially in relation to ATP and CFU count, which showed statistically significant reductions in stages II and III. The authors of the ECU study point toward the need to provide continuing training and constant feedback to the team as a strategy to maintain cleaning efficacy [32].
Furthermore, in a specialized healthcare unit from the Brazilian Midwest region, after the educational intervention, the success rates increased by 43.96% (ATP) and 12.46% (CFU) in phase I, by 70.6% (ATP) and 82.3% (CFU) immediately after the intervention, and by 76.52% (ATP) and 85.76% (CFU) two months after intervention [30]. The current study in a pediatric unit also obtained positive results in relation to the approval percentages, which were increased in stage II, showing that all surfaces had an approval rate of 100% for ATP, except for the companion's armchair, which presented 62.5% approval. In stage III, the approval rates were 100% for two surfaces (toilet flush handle and bathroom door handle), 87.5% for one surface (bed rail), and 75% for another surface (companion's armchair).
The current study, as well as those cited in this research, reinforce the pressing need to periodically monitor and assess the CSS procedure, in order to ensure its consistency and proper execution, in addition to the need to provide training to all the professionals based on basic and practical precautions to prevent HAIs.
The efficacy of the educational interventions is related to various aspects involving characteristics of the services, material and human resources, team engagement, audits, and feedback, among others. A study conducted in Australia in 11 hospitals, with a cleaning bundle related to a multimodal intervention, including proper use of products and technique, as well as training and feedback for the team, added to auditing and communication, allowed reducing infections by vancomycin-resistant enterococci and increasing CSS effectiveness [35].
The impact and sustainability of an educational program with the environmental cleaning team were evaluated in five hospitals from a network in New York City, USA. The five-module program sought to address previously identified gaps in knowledge, attitudes, and practices related to infection prevention and provide strategies to reduce or eliminate challenges and barriers often found by environmental hygiene teams when developing their work. The evaluations took place daily in patients' rooms, before implementation of the educational program (no intervention), approximately three months after the end of the program (short-term assessment of the intervention), and one year after the educational program (long-term assessment of the intervention) by means of ATP detection (<250 LRUs). The educational program was found to promote significant improvements in CSS monitored in the short- and long-term, in addition to favoring a significant reduction in the rates of hospital infections by Clostridium difficile [36].
It is important to highlight that implementation of a training program requires adequate standardization of products and supplies, definition of disinfectant concentration, amount and proper contact time with the surface, and application method [37, 38], ensuring uniformity in the CSS process and, thus, a reduction in microbial density, organic matter and possibly, HAIs.
It is noted that, in the current study, environmental sampling was done by the same person both before and after CSS. This is advantageous because the researcher attempted to maintain the sampled areas before and after sample collection. On the other hand, the process depended solely on memory, as the areas were not marked. However, small changes in the position of the plates and ATP swabs in the collection before and after CSS may have occurred.
A study conducted with the objective of assessing the efficacy of the improvement in surface cleaning to reduce environmental bacterial load in a neonatal intensive care unit in Morocco showed that the incorporation of improvements in the CSS process led to a significant reduction in the CFU count and to the absence of microorganisms as early as two days after the educational intervention, evidencing the importance of continuing education of the health professionals and hygiene and cleaning workers. Such improvements in the CSS process consisted of the inclusion of a checklist for monitoring the surfaces, concern about the workers' clothing hygiene, use of disposable wipes instead of reusable cloths for surfaces, floors, and walls, maintenance by the contact time of the disinfectants, and for the equipment, use of disposable wipes containing didecyl dimethyl ammonium chloride and polyhexamethylene biguanide hydrochloride [39].
An aspect that warrants further research studies is the way to apply the disinfectant after implementing the SPCSS since, according to its manufacturer, the sanitation product has a residual effect from quaternary ammonium for 72 hours after its application. Therefore, the use of microfiber, together with a possible improvement in friction, may have exerted an influence on the decreased microbial density of the surfaces and ATP quantification. Regarding this aspect, it is worth noting that, before the SPCSS, the cloth used was made of cotton, and in this regard, a study by Engelbrecht [40] showed that, due to the presence of cellulose in cotton cloths, the quaternary ammonium concentrations were reduced by up to 85.3%, resulting in failure of the disinfectants based on this sanitizer, due to its bond to cellulose.
With regard to the long-term monitoring of the CSS process, some studies [30, 32] showed that the results were significant in four of the five surfaces evaluated by means of the ATP detection method, with no significant variation in ATP (LRU) or CFU count, suggesting that the positive results of the interventions did not last in the long term when it comes to CSS efficiency related to CFU count reduction. In the current research, although it was observed that the results remained statistically significant for the four surfaces in the long term, it is important to note that stage I was the one that presented the highest number of approved surfaces in terms of reduced CFU/cm2, albeit with a low percentage (Fig. 2), also showing the challenge of maintaining positive results in the long term.
It is noted that naming the evaluation of interventions as short- medium- and long-term both in the studies described and in the current research represents arbitrary random dates, as no study analyzed includes any theoretical framework to establish these periods of time. Therefore, it is not possible to ignore that different CSS monitoring times do not exert an impact on the results of the indicators used to monitor the CSS process in the studies. Moreover, the comparisons between these study designs are complex, considering not only their time frames but also other factors, such as brands of products and equipment for CSS monitoring, cutoff point to consider a surface as clean, conditions and types of sampled surfaces, sanitizers and their ways of application, types of fabrics used, CSS frequency, professionals in charge of this process, content, time, and methodologies used in the educational interventions.
With regard to the visual inspection, in statistical terms, only the bathroom door handle (stages I and III) and the toilet flush handle (stages I and III) passed the test. However, on visual inspection, all surfaces, except for the bed rail in stages II and III, presented higher approval percentages before and after CSS in all three study stages. For the same monitoring method, a study [32] also observed a progressive reduction in the number of surfaces considered inadequately cleaned and disinfected from phase I to phase III. Another study conducted in an Emergency Unit also observed an increase in the approval proportions for the visual method from 25.0% to 100% in phase II, and from 25.0% to 87.5% in all the areas evaluated in phase III [19].
It is pertinent to highlight that some items of furniture did not pass the test due to defects in their physical structure (such as scratches and tears), which often end up requiring repairs that go beyond the governance power of the professionals directly in charge of the CSS process and of the nursing team, a situation that was much present in the structure of the companion's armchair. In addition, the items of furniture are not uniform in their physical and hygiene integrity across the rooms evaluated, in addition to the fact that the collections were performed in the morning period when the items usually had not undergone CSS for a long time, which can explain these results.
Another determining factor for the failure to reach the best parameters in microorganism reduction and ATP can be related to the presence of biofilm on these surfaces. The presence of biofilm on frequently touched surfaces in healthcare environments raises questions about its role in the transmission of pathogens that eventually cause hospital infections [41]. It also raises questions on the efficacy of removal and inactivation of microorganisms within the biofilm by the cleaning and sanitation methods currently used on the surfaces of healthcare environments, indicating that the biofilm probably acts as an environmental reservoir for microorganisms, even when it is partially removed [42].
Furthermore, it is not possible to ignore the possibility that the nursing team may not have performed CSS on the bed rail, or performed it less efficiently and frequently. This fact can have several explanations, such as lack of time, valuing of patient care to the detriment of CSS, routine of administrative procedures, patient turnover, demand for beds, and availability of the nursing professionals who are in charge of cleaning this surface and need to be present when CSS is performed by the HCTPs.
The current study emphasizes that all surfaces had approval rates above 50% in all the study stages when assessed by means of the ATP bioluminescence method, corroborating other studies [30-32] that used similar methodologies.
It should be emphasized that adherence to the PPLDS contributes to avoiding environmental contamination. In this regard, carrying out adequate cleaning allows an effective contribution to reducing the occurrence of HAIs [43, 44] (Dramowski, et al., 2021; Mitchell et al., 2020). L&D practices have always been important for controlling environmental contamination, and during the COVID-19 pandemic, there was even more of a process of greater significance and intensity, in which performing L&D assertively reduces the potential for the spread of microorganisms, including viruses. It is essential to establish adequate hygiene protocols and measures [45]. A study demonstrated the presence of COVID-19 on environmental surfaces close to patients with COVID-19, such as (bedside tables, remote control, bed rails, and floor [46].
We, therefore, suggest that a combination of the CSS monitoring methods will provide more reliable data regarding the evaluation of the efficacy of this process. Additional studies are necessary to evaluate and refine these standards in order to measure the CSS frequency required for a specific area or to change the protocol or material used in this process.
4.1. Study Limitations
The limitations of this study are related to the analysis of CSS only in a single institution, the limited period for data collection, and the fact that it was performed in only one period of the day, although the unit studied is a reference for the hospitalization of children from several municipalities. The sample size was small, and some surfaces of the rooms were not included due to a scarcity of financial resources. There was also the possibility of the Hawthorne effect since, despite attempts to minimize it in stage I, some professionals might have been inclined to improve their performance; in addition, the scarce literature on the evaluation of CSS efficacy in pediatric hospitalization units restricted comparisons. Another fact is the non-association of the intervention implemented with reductions in the colonization and/or infection rates of the children treated in this unit.
CONCLUSION
This study contributed to the impact of implementing a Surface Cleaning and Disinfection Standardization Program (PPLDS) in a pediatric hospitalization unit during the COVID-19 pandemic period. An improvement in the rates of cleaning and disinfection of surfaces after the intervention was reported. In this regard, the research is presented as a subsidy in the implementation of educational actions and protocols together with health professionals that aim to minimize environmental contamination.
The importance of investing in the maintenance and conservation of the physical structure of furniture in pediatric hospitalization services was also highlighted, as this characteristic impacts the effectiveness of the cleaning and disinfection process. We also suggest that nursing training programs should allow continuous monitoring, with feedback on results and the use of suitable products for performing LDS, which will allow satisfactory results. New research should be carried out seeking to monitor the effect of PPLDS on the indecency of nosocomial infection rates in the pediatric context.
LIST OF ABBREVIATIONS
| MRSA | Methicillin-resistant Staphylococcus Aureus |
| VRE | Vancomycin-resistant Enterococcus |
| SPCSS | Standardization Program for Cleaning and Sanitation of Surfaces |
| SOP | Standard Operating Procedure |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethics research committee of the federal university of Mato Grosso do Sul, Brazil (CAAE: 29350219.8.0000.0021).
HUMAN AND ANIMAL RIGHTS
No animal were used that are the basis of this study. The study development complied with national and international standards of research ethics.
CONSENT FOR PUBLICATION
Informed consent was obtained from all the participants.
AVAILABILITY OF DATA AND MATERIALS
All the data and supportive information are provided within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support of Coordination for the Improvement of Higher Level Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES - Brazil) and of the Federal University of Mato Grosso do Sul and FUNDECT - Foundation to Support the Development of Education, Science and Technology of the State of Mato Grosso do Su.


