SYSTEMATIC REVIEW
The Effectiveness of Care Bundles Including the Braden Scale for Preventing Hospital Acquired Pressure Ulcers in Older Adults Hospitalized in ICUs: A Systematic Review
Natalie A. Floyd1, 2, 3, Karen A. Dominguez-Cancino4, 5, 6, Linda G. Butler7, Oriana Rivera-Lozada5, 8, Juan M. Leyva-Moral5, 9, Patrick A. Palmieri1, 5, 10, 11, *
Article Information
Identifiers and Pagination:
Year: 2021Volume: 15
First Page: 74
Last Page: 84
Publisher ID: TONURSJ-15-74
DOI: 10.2174/1874434602115010074
Article History:
Received Date: 25/08/2020Revision Received Date: 1/2/2021
Acceptance Date: 7/2/2021
Electronic publication date: 20/04/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Despite technological and scientific advances, Hospital Acquired Pressure Ulcers (HAPUs) remain a common, expensive, but preventable adverse event. The global prevalence ranges from 9% to 53% while three million people develop HAPUs in the United States and 60,000 people die from associated complications. HAPU prevalence is reported as high as 42% in ICUs (ICU) costing on average $48,000 to clinically manage.
Objective:
The purpose of this systematic review was to evaluate the effectiveness of multi-component interventions (care bundles), incorporating the Braden scale for assessment, in reducing the prevalence of HAPUs in older adults hospitalized in ICUs.
Methods:
This was a systematic review of the literature using the Cochrane method. A systematic search was performed in six databases (CINAHL, Cochrane Library, Google Scholar, JBI Evidence-Based Practice Database, PubMed, and ProQuest) from January 2012 until December 2018. Bias was assessed with the Critical Appraisal Skills Programme Checklist, and the quality of evidence was evaluated with the American Association of Critical-Care Nurses Levels of Evidence.
Results:
The search identified 453 studies for evaluation; 9 studies were reviewed. From the analysis, pressure ulcer prevention programs incorporated three strategies: 1) Evidence-based care bundles with risk assessments upon admission to the ICU; 2) Unit-based skincare expertise; and 3) Staff education with auditing feedback. Common clinical management processes included in the care bundles were frequent risk reassessments, daily skin inspections, moisture removal treatments, nutritional and hydration support, offloading pressure techniques, and protective surface protocols. The Braden scale was an effective risk assessment for the ICU. Through early risk identification and preventative strategies, HAPU programs resulted in prevalence reduction, less severe ulcers, and reduced care costs.
Conclusion:
Older adults hospitalized in the ICU are most vulnerable to developing HAPUs. Early and accurate identification of risk factors for pressure is essential for prevention. Care bundles with three to five evidence-based interventions, and risk assessment with the Braden scale, were effective in preventing HAPUs in older adults hospitalized in intensive care settings. Higher quality evidence is essential to better understanding the impact of HAPU prevention programs using care bundles with risk assessments on patient outcomes and financial results.
1. INTRODUCTION
Despite technological and scientific advances, Hospital-Acquired Pressure Ulcers (HAPUs) are among the top five most common causes for adverse patient outcomes [1, 2]. For years, the global HAPU prevalence has ranged from less than 1% to more than 40% [3], with a mean prevalence of 14.8% [4]. In American hospitals, the prevalence is estimated to be 0.4% to 38% [5]. Each year, nearly 3 million people develop HAPUs [6] in the United States, costing $10 billion with a $48,000 average charge per ulcer [7]. More than 60,000 acute care patients die from complications related to HAPUs [8], yet as many as 95% are preventable [7]. For this reason, Hibbs [9] noted HAPUs are ‘an epidemic under the sheets.’
Pressure ulcers are a serious but common adverse event for older adults [10]. Within one week of hospital admission, about 15% of patients more than 60 years old will develop a pressure ulcer [11], with an average unadjusted inpatient cost of $66,064 versus $35,844 for patients with and without HAPUs [12]. The additional hospital stay related to a HAPU is on average 4 to 6 days, reducing the availability of beds for other admissions [13]. Hospital payments for managing HAPUs were eliminated in the United States [14], resulting in the financial incentive for risk reduction programs [15]. With hospital-wide implementation of evidence-based practices, HAPUs can be significantly reduced (11.32 cases/quarter) [16].
In the intensive care unit (ICU), HAPU prevalence is reported between 4% to 40% [17], with higher prevalence reported in the medical ICU [18]. HAPUs in the ICU contribute to increased nurse workload, as high as 50%, with at least a 5% impact on the overall budget [19] due to more staffing, medical supply consumption, specialty bed usage, and nutritional support [20]. HAPUs have a deleterious impact on the quality of life for people recovering from illnesses with limited mobility, increased incidence of sepsis, additional surgeries and extended hospital stay [21, 22]. For these reasons, HAPUs are a recognized quality of care surrogate, risk management problem, and patient safety priority for the ICU [17, 23].
1.1. HAPUs and Nursing
Classified as a nursing-sensitive quality indicator [24], HAPUs are acknowledged to be a fundamental nursing responsibility in the ICU [25, 26]. The three independent predictors for HAPU development are generally managed through nursing services in the hospital setting including, mobility and activity, perfusion related to diabetes and other diseases, and skin integrity, including pressure ulcer status [27]. As HAPUs can develop within four to six hours [28], nurses can prevent ulcers by identifying high-risk patients, initiating evidence-based intervention strategies, and monitoring for signs of ulcer development [29]. Pressure ulcer prevention programs have improved with multidisciplinary teams [30] led by nurses as the most knowledgeable about HAPUs, capable of identifying high-risk patients, and available to implement recommended interventions [31].
1.2. Care Bundles and Risk Assessment
Although intervention studies to prevent HAPU development have been conducted in different clinical settings, most employed single interventions in comparison to standard care [32]. However, multi-component interventions, or care bundles, with a risk assessment are more effective in preventing HAPU development [29, 33]. The Braden scale for predicting pressure sore risk [34-37], more commonly called the Braden scale [38], is the most widely used tool in hospitals [39] to identify patients at high risk for HAPUs. The Braden scale is highly effective in assessing HAPU risk among patients in medical, surgical, and critical care settings [40], and is more accurate than the clinical judgement of nurses [41].
Care bundles combine evidence-based interventions, usually three to five components, to yield a significantly better outcome than when individually implemented [42, 43]. To maximize the clinical outcome, all the interventions must be performed collectively and implemented consistently [44]. Most HAPU care bundles include a risk assessment, support surfaces, patient repositioning, mobilization, friction reduction, nutritional support and moisture management [45]. Additional intervention strategies include unit-based wound care clinicians, health record monitoring, audit result feedback, staff education, computerized processes, and standardized clinical practices [6].
1.3. Purpose of Systematic Review
Knowledge is translated from research into clinical practice [46] based on the evidence reported by systematic reviews [47, 48]. Yet, there has not been a systematic review of the intensive care literature published since 2002 for HAPU prevention strategies incorporating the Braden scale [49], and none focused on care bundles. The purpose of this systematic review was to evaluate the effectiveness of care bundles incorporating the Braden scale for risk assessment in reducing the HAPU prevalence in older adults hospitalized in the ICU.
2. MATERIALS AND METHODS
This systematic review was guided by the methodological framework outlined by the Higgins & Green [50] through ten steps, including: 1) Frame research question; 2) Construct search strategy; 3) Test search strategy in PubMed; 4) Identify relevant studies for sample; 5) Assess the level of evidence; 6) Evaluate the risk for bias; 7) Extract data from studies; 8) Summarize the data; 9) Interpret the findings; and 10) Report the evidence [51-53]. The PICOS (participants, interventions, comparators, outcomes,and study design) technique [54], with the addition of time (PICOTS), defined the searchable research question (Supplemental Table 1). The study was approved by the university institutional review board and reported according to the preferred reporting items for systematic reviews and meta-analyses, or PRISMA [55].
2.1. Search Strategy
A systematic search was performed using thesaurus terms and keywords in six databases, CINAHL, Cochrane Library, Google Scholar, JBI Evidence-Based Practice Database, PubMed, and ProQuest between January 2012 and December 2018 for research papers reporting experimental, quasi-experimental, and observational study designs. For the search strategy, a combination of search terms and keywords included: pressure ulcer, Braden scale, ICU, pressure ulcer prevention, protocol, intervention, multi-component, and care bundle, combined with Boolean operators (Supplemental Table 2). The inclusion criteria were research studies with three or more interventions (care bundle), male and/or female patients 60 years of age or older, hospitalized in the ICU for at least 24-hours, and without HAPUs upon entry into the unit.
The review leader conducted the pilot search in PubMed, before initiating the searches in six databases with a second reviewer. The search strategy for each database, with keywords, was shared with the second reviewer to ensure the comprehensive search could be repeated in a substantially similar manner. An additional reviewer, an expert in scoping and systematic reviews, provided guidance throughout this process, including testing the search strategy with a qualified biomedical reference librarian.
2.2. Study Selection
Once the search was completed, the titles, abstracts, and full papers were independently assessed by two reviewers in sequential rounds applying the inclusion criteria. The PRISMA [54] four-step process for study review and selection was utilized: a) identification (records identified), b) screening (titles reviewed and abstracts screened), c) eligibility (full-text assessed) and d) included (document included in the sample). When there was not agreement between the two reviewers during the title and abstract screening, the study was included in the next round for further consideration to reduce the opportunity for selection bias. To prevent methodological bias, the Critical Appraisal Skills Programme (CASP) Checklist [57-59] (qualitative, randomized control, case-control, cohort, or systematic review) was applied to assess the quality, relevance, and results of each study. At least 70% of the criteria for each checklist needed to be met for inclusion. Any uncertainty or disagreements were collaboratively resolved through a review of the questionable criteria to reach a consensus.
2.3. Data Collection and Analysis
After the sample was defined and the study quality evaluated, data from the included studies were abstracted into an Excel-based literature review matrix for analysis and synthesis. The data abstraction was completed by two reviewers and verified by a third. The quality of evidence was evaluated with the American Association of Critical-Care Nurses Levels of Evidence [60]. Studies assessed within the first four evidence levels (A, B, C, and D) were included in the review, while level M studies were excluded. Studies assessed as the highest level of evidence, or level A, include a meta-analysis of multiple controlled studies or meta-synthesis of qualitative studies with results that consistently support a specific action, intervention, or treatment. The next level, or level B, describes well-designed controlled studies, both randomized and non-randomized, with results that consistently support a specific action, intervention, or treatment. Then, level C studies are qualitative, descriptive, correlational, integrative reviews, systematic reviews, or randomized controlled studies with inconsistent results. Next, level D studies include peer-reviewed professional organizational standards, with clinical studies to support recommendations. Finally, level M are manufacturer recommendations. This leveling facilitated study comparison to identify the strongest evidence for clinical practice [61].
3. RESULTS
From the 453 papers included in the title review, the full texts of 43 studies were reviewed with 34 studies [23, 28, 30, 32, 33, 45, 62-89] excluded (Table 1). The final sample of nine studies [90-98] (Table 2) met the CASP checklist threshold for inclusion. The complete sampling process is provided in the PRISMA diagram (Fig. 1). Most data extracted for this review originated from studies assessed at level C (n=7), with 1 study at level B and 1 at level D. The studies largely reported quality improvement programs with outcomes focused on care bundles with some addressing implementation strategies such as unit-based expertise in wound care and staff education with audit feedback.
| Author & Year | Rationale for Exclusion |
| Bergstrom et al. (2013) | Single intervention study conducted in nursing homes. |
| Black et al. (2012) | Single intervention study conducted in a 12-bed cardiovascular ICU. |
| Chaboyer et al. (2016) | Sample of medical and surgical patients; no standard risk assessment, Braden scale not used. |
| Chou et al. (2013) | Single intervention comparative in three ICUs; Braden scale as a risk assessment was not a focus. |
| Coladonato et al. (2012) | Single intervention study with aggregated data; intervention not specific to ICU patients. |
| Cooper (2013) | Study reviewed the development of pressure ulcer protocols; not an intervention study. |
| Cowan et al. (2012) | Explored the use of the Braden scale and risk assessments; not an intervention study. |
| Cox (2011) | Risk assessment discussion without an intervention. |
| Coyer et al. (2015) | Before and after study design with control group, skin integrity bundle intervention; Braden scale not used. |
| Dutra et al. (2015) | Single intervention comparative study with dressing changes. |
| Edger (2017) | Single intervention comparative study conducted in the neonatal ICU. |
| Estilo et al. (2012) | Discussed the development of a protocol; not an intervention study. |
| Evans et al. (2013) | Discussed care bundles for pressure ulcer prevention for different clinical areas; not an intervention study. |
| Flike (2013) | A case study with the Braden scale, not an intervention study. |
| Gillespie et al. (2014) | Single intervention comparative review of repositioning and pressure ulcers; not limited to the ICU. |
| Guihan et al. (2014) | The study was primarily limited to the ICU; younger spinal cord injury patients admitted for severe pressure ulcers. |
| Hall et al. (2016) | Single intervention study focused on a turn-and-assist device and nursing time in the ICU; age was not discernible. |
| Krupp et al. (2015) | Literature review about the prevention and management of pressure ulcers in the ICU; not an intervention study. |
| Mallah et al. (2014) | Study included patients from medical–surgical, oncology, pediatrics, and ICUs, data aggregated, not detailed. |
| Myers (2017) | Single intervention study with heel protector and pillows; results are not separate by different types of units. |
| Niederhauser et al. (2012) | Primarily single and double intervention studies; one from an ICU setting in 2008; data not reported specific to ICU. |
| Ozyurek & Yavuz (2015) | Single intervention study comparing viscoelastic versus standard hospital foam. |
| Park et al. (2017) | Single intervention study comparing foam overlay and standard hospital mattress; multiple unit data aggregated. |
| Ranzani et al. (2016) | Study conducted in multiple ICUs but focused on pressure ulcer prediction; there was no bundled intervention. |
| Smith et al. (2013) | Single intervention study comparing standard hospital and synthetic linens; telemetry, urology, and ICUs. |
| Tayyib & Coyer (2016) | Adult ICU participants; focused on effectiveness of single strategies; risk assessment not specific to Braden Scale. |
| Tayyib et al. (2016) | Described implementation of a pressure ulcer care bundle measured in a companion study; not an intervention. |
| Tescher et al. (2018) | Cohort study of treatment using electronic records; not an intervention study; specific age groups not reported. |
| Thorpe (2016) | Single intervention study focused on dressings; Braden scale was not used. |
| Twersky et al. (2012) | Single intervention study conducted in a nursing home. |
| Yap et al. (2011) | Discussed nurse-led approaches to reduce pressure ulcers; not an intervention study. |
| Yap et al. (2016) | Discussed the process of cuing to facilitate staff pressure ulcer program implementation in a nursing home. |
| Webster et al. (2011) | Waterlow or Ramstadius assessments used in study; range of patients from the medicine and oncology units. |
| Zuo et al. (2015) | Discussed the development of an evidence-based care bundle; not an intervention study. |
|
Author & Year |
Study Design |
Age Group |
Intervention(s) |
Outcomes / Results |
Evidence Level |
| Padula et al. (2016) | Retrospective observational cohort. | Four age groups: 18–30; 31–50; 51–64; & > 65 years. | Braden scale with staff strategies, information technology, and performance improvement. | Pressure ulcer prevention protocol led to a 27% reduction or 1.8 few HAPU cases per quarter. | C |
| Swafford et al. (2016) | Quality improvement, with chart review. | Mean age in 2011: 51.9; 2012: 50.5; 2013: 59. | Braden scale, skin care, fluidized repositioners, silicone adhesive dressings, and staff education. | HAPU incidence decreased by 69 (n = 17; 3% of patients in 2013 vs n = 45, 10% of patients in 2011) with 22% in patient load. | C |
| Anderson et al. (2015) | Pre- and post-intervention design. | Mean age was 62.71 years (17.12) SD. | Prevention bundle with Braden Scale, skin emollients, heel protection, and repositioning. | HAPU incidence decreased from 15.5% to 2.1%. Multivariate logistic regression model showed a significant reduction in HAPU (P<.001). | C |
| Cano et al. (2015) |
Quality improvement, with chart review. | Most of sample (81%) was 50+ years. | Braden scale, support surfaces, skin assessment, repositioning, skin barrier products, WOC nurse. | HAPU dropped to 2.6% in two quarters and remained between 1% and 2% for 9 quarters. | C |
| Qaseem et al. (2015) | Systematic review with clinical guideline. | Various ages but included many older adults. | Braden scale, mattresses, repositioning, dressings, barrier creams, and education. | Three recommendations were developed for a clinical practice guideline. | D |
| Tayyib et al. (2015) | Prospective cohort study with control group. | Mean age 50 years, with range between 18–99. | Risk and skin assessments, skin care, nutrition, repositioning, support surface, and education. | Age, length of stay, cardiovascular and kidney diseases, infrequent reposition, emergency admission, mechanical ventilation, and lower Braden scale scores predicted HAPU. | B |
| Armour-Burton et al. (2013) | Quality improvement, with chart review. | Various ages but included older adults. | Braden Scale, skin assessments, use of pressure reducing mattress, and 2-hour repositioning. | After implementation of Healthy Skin Project, the prevalence decreased from a mean of 4.85% to 0% for 17 of 20 quarters. | C |
| Sullivan et al., (2013) | Systematic review with 26 studies (18 acute care). | Various ages but included older adults. | Braden scale, support surfaces, repositioning, moisture management, and nutritional assessments. | Some improvements in HAPU rates in 24 studies, significant findings in 11 (13 no significant finding); 5 reported improvement HAPU rates. | C |
| Kelleher et al., (2012) | Quality improvement with chart review. | Mean age ranged from 53.3 to 60.7 years. | Braden Scale, moisture prevention, skin and nutrition assessments, and support surfaces. | The highest prevalence was 27%; after interventions, HAPR rates reported 1% for 3 consecutive quarters. | C |
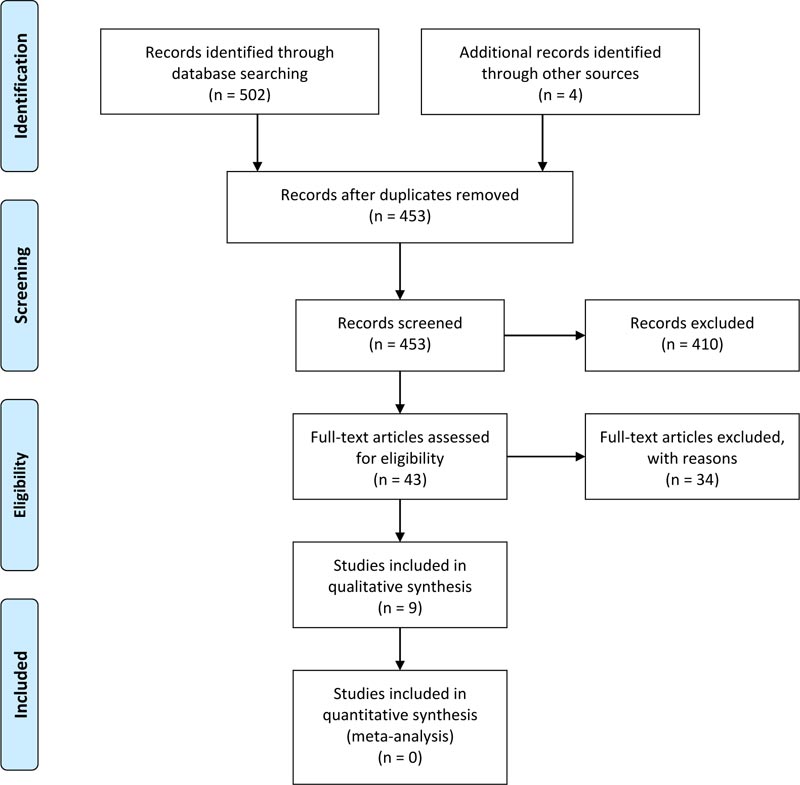 |
Fig. (1). PRISMA 2009 Flow Diagram. |
3.1. Hospital Acquired Ulcer Prevention Programs with Care Bundles
In a systematic review comparing intervention effectiveness for a HAPU clinical practice guideline, Qaseem et al. [95] reported care bundles significantly improved skin care and reduced HAPU rates, with a cost savings of at least $3,000 per case. Similarly, Sullivan & Schoelles [96] reported care bundles significantly reduced HAPU rates in 11 (42%), with a mean reduction of 82% (range 67% to 100%). For assessment within the care bundles, there was also no significant difference reported in diagnostic accuracy between the Braden, Cubbin and Jackson, and Norton and Waterlow scales [95].
The single randomized controlled trial (n=140) was reported from Saudi Arabia [98]. In this study researchers compared a care bundle (risk and skin assessments, skincare, nutrition, repositioning, and support surfaces) with a training program for the intervention group to normal care for the control group. HAPU incidence was significantly reduced in the intervention group (7.14%, 5/70 patients) when compared to the control (32.86%, 23/70 patients) [98]. There was also significantly less stage I/II pressure ulcers, with no stage III/IV, development for the intervention group. Differences in care processes were also observed for repositioning (85% every three hours for the intervention group compared to 20% every two hours for the control group) and health protector application (97% for the intervention group compared to 0% control group). Furthermore, a retrospective observational cohort study found there was a longitudinal impact of payment policies on the quality improvement interventions to prevent HAPUs. In this regard, Padula et al. [94] observed hospitals adopting bundled interventions had a 27% reduction in HAPUs (-1.86 cases/quarter; p=0.002). The bundled interventions were attributed to changes in reimbursement policy, resulting in a 100% reduction in HAPU cases (-11.32 cases/quarter, p<0.001).
3.2. Unit-Based Wound Care Expertise
Three studies [90, 92, 93] reported pressure ulcer prevention bundle implementations with the inclusion of a wound, ostomy, and continence (WOC) clinician and/or skincare champion. In a pre- and post-intervention design, Cano et al. [92] evaluated a multidisciplinary HAPU quality improvement program with an evidence-based protocol, staff education, WOC nurse, and environment of care improvements such as new inpatient support surfaces. The prevalence decreased from 11.7% (stage 2 to 4 ulcers) to 2.1% after program implementation. As the prevalence rose to 5.1% across several quarters, an additional staff education resulted in the continued reduction to 2.8% for 10 consecutive quarters. The program with evidence-based practices, protective products, and staff education reduced the HAPU risk in the short-term, and repeated staff education resulted in the sustained reduction.
In a quasi-experimental pre- and post-intervention study, Anderson et al. [90] compared a universal pressure ulcer prevention bundle and semi-weekly WOC nurse rounds with standard care in a sample of ICU patients (n=327). While the prevention bundle included the same components as the standard care, the interventions differed in length, complexity, number of interventions, and accessibility. Statistically significant differences were observed between the two groups for the pre- and post-intervention results specific to repositioning and elevation of heels for the prevention bundle group. The prevention bundle with expert unit-based wound care was most effective in reducing the HAPU risk.
After experiencing a 27% HAPU prevalence, Kelleher et al. [93] implemented a 36-month quality improvement program in a 17-bed surgical ICU. The average patient age for HAPUs was 57.9 ± 16.7 years, with a mean Braden scale score of 13 ± 1.2 (range, 9-17). The underpinning of the intervention was monitoring Braden subscale scores. Also, peer-to-peer interactions, skin care champion, and a WOC nurse were incorporated to facilitate teamwork and to provide unit-based expertise. During the implementation phase, prevention surface utilization increased 92%, repositioning increased 30%, nutrition assessments increased 77% and moisture management increased 100%.
3.3. Staff Education with Audit Feedback
From a systematic review of interventions, Sullivan & Schoelles [96] reported successful quality improvement programs with care bundles reporting reduced HAPU risk almost always included staff education (25/26 studies) and frequently included staff feedback from audits (12 of 26). In addition, Swafford et al. [97] assessed the effectiveness of a year-long HAPU prevention program in an adult ICU, including the Braden scale, revised skin-care protocol, fluidized repositioners, silicone adhesive dressings, and face-to-face staff education.There was a 69% reduction in HAPUs at the end of the program (45 HAPUs among 10% of patients before and 17 ulcers among 3% of patients after). The authors reported staff education with performance feedback positively contributed to the program outcomes. Similarly, Armour-Burton et al. [91] reported a multidisciplinary healthy skin project eliminated HAPUs in a surgical progressive care unit (mean of 4.85% to 0% for 17 quarters). The key intervention strategies were staff education, unit based WOC nurse, risk assessment (Braden scale) with the normal care (skin assessments and repositioning, specialty mattresses and dressings, and nutrition support). Finally, Kelleher et al. [93] attributed the elimination of HAPU (from a mean of 27% to 0% for three consecutive quarters) to enhanced education and feedback provided by a WOC nurse. Overall, quality improvement programs with multidisciplinary participation, structured education, and adherence to evidence-based protocols resulted in significant HAPU reductions [91, 93, 97].
4. DISCUSSION
Critically ill people hospitalized in the ICU are more likely to develop a HAPU than other hospitalized people. Older adults are more significantly at risk for serious HAPUs with a more problematic ulcer profile in terms of prevalence, stage, and location. As people with HAPUs have more complications, such as pneumonia and renal failure [99], programs often focus on achieving cost reductions based on reforms in hospital reimbursement rather than clinical outcomes [94]. Despite evidence indicating the hospitals are responding to the reimbursement problem with quality improvement initiatives with evidence-based strategies to identify high-risk patients to prevent ulcer development, program comparison has been obstructed by the lack of uniformity in terms, inconsistent concepts, and dissimilar measurements [100].
The quality of evidence was substantially limited by the mostly single site quality improvement study designs. However, programs incorporating care bundles have been reported to be more effective than single interventions in reducing HAPUs in multiple studies [45, 92, 101]. The care bundle benefits seem to be derived from the synergy created by the different interventions. For example, including a sub epidermal moisture measurement in a care bundle results in the identification of early skin damage four days sooner than a nurse assessment [102]. This is why care bundles have been described as the standard of care to prevent HAPUs [95].
With the care bundles, there are many other changes to note about program effectiveness. In addition to care bundles, hospitals reported educating and training staff, revising assessment protocols, enhancing wound documentation, implementing quality audits with feedback for staff, adopting the Braden scale, and redesigning reporting processes are also important strategies to reduce HAPUs [96]. As the practice setting can influence nursing decisions, the effective programs incorporated teams facilitating the mutual support between nurses and other professionals. Multidisciplinary team success resulted from planned collaboration and effective communication. This may explain the missing relationship between years of practice experience and academic preparation with care bundle adherence [103]. Critical care nurses are often skin care ‘champions’, while WOC nurses, dieticians, and physical therapists are supportive team members.
Educational interventions are not only necessary for implementing care bundles but also for improving diagnostic accuracy and reducing misclassifications with the HAPU risk assessment [104]. Due to inaccuracies in diagnosing, classifying, and reporting pressure ulcer injuries, international guidelines are needed to support standardized clinical assessments, data reporting, and outcomes management [105]. Similarly, educational interventions are essential to reliably identify and classify HAPUs [106] and to assess the accuracy of clinical definitions and classification systems [107]. Standardized programs are an essential strategy to strengthen the clinical comparisons necessary to assess HAPU interventions.
HAPUs remain a leading patient safety problem in the intensive care setting [108] despite new technologies and continued quality improvement projects. For this reason, large multicenter ICU studies, incorporating an experimental design with evidence-based interventions, such as care bundles, including the Braden scale, multidisciplinary teams, evidence-based protocols, and clinical education, need to be undertaken to advance the HAPU science. These studies not only need to report prevalence and patient outcome data, but also explore the benefits afforded by technology to minimize the context of burdensome clinical workload with reliable reminders about ulcer care [109]. Finally, new studies need to focus on data mining in data-rich health systems to develop predictive models to compliment risk assessments, such as the Braden scale, to reduce HAPUs in targeted populations [110].
4.1. Strengths and Limitations
There are six limitations for this systematic review which also serve as recommendations for future researchers. First, there was only one study representing the highest levels of evidence. As most PU prevention interventions were designed as single site quality improvement projects, the study design was primarily before-and-after [29]. Second, the scope of this review was limited to bundled interventions incorporating the Braden scale. This limitation strengthened the review by comparing studies using the same risk assessment with similar interventions. However, studies with stronger evidence but not incorporating the Braden scale could have been excluded. Third, two systematic reviews were included in this review as they addressed complex interventions, care bundles, and organizational features for program implementation, such as staff education. However, the results clearly state the context of these reviews to minimize the potential for bias when weighing the evidence. Fourth, methodological heterogeneity [111] was observed with fundamental differences in study designs, prevalence and incidence reporting, and data collection methods that limited the systematic comparisons for a more robust synthesis. In this regard, standards for measuring and reporting need to be implemented to facilitate more opportunities for comparative HAPU prevention research [112]. Fifth, the largely positive study results observed in this review may be attributed to publication bias [33]. Finally, the sample of studies were primarily reported from the United States which might limit the generalizability of the findings. Despite these limitations, this review addresses a wide gap in the literature specific to HAPU prevention programs and the outcomes of older adults hospitalized in the ICU. This review provides important knowledge to guide the development of replicable quality improvement projects with similar research designs, variable definitions, and intervention strategies.
CONCLUSION
Most HAPU reduction programs are implemented as quality improvement studies with a before-and-after design in a single ICU. However, the available evidence suggests early identification of pressure ulcer risk factors with rapid implementation of mitigation strategies in the form of care bundles reduces HAPUs and decreases health care costs. The Braden scale was most widely used risk assessment and reported to have the highest predictability in ICUs. The most effective programs incorporated care bundles with multidisciplinary teams, education and training, unit based wound care expertise, and audit feedback to clinicians. There is a lack of multicenter research studies with robust experimental designs using standardized processes, recognized measurements, and uniformed reporting strategies. Randomized controlled trials are essential for evaluating care bundle efficacy and organizational process effectiveness across ICUs.
LIST OF ABBREVIATIONS
| CASP | = Critical Appraisal Skills Programme |
| HAPU | = Hospital Acquired Pressure Ulcer |
| ICU | = ICU |
| PRISMA | = Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| WOC | = Wound, Ostomy, and Continence |
AUTHORS’ CONTRIBUTIONS
All authors participated in the manuscript submitted for journal review. The following authors were involved in the stated phases of the project: Study conception (NAF); study design (NAF, PAP); data collection (NAF, KAD, LGB, PAP); data analysis (NAF, KAD, LGB,); data interpretation (); drafting the manuscript (NAF, LGB, JML, PAP); table development (NAF, KAD, ORL, PAP); substantial revisions to manuscript (PAP, JML, KAD), critical revisions to final manuscript (NAF, PAP, JLM, ORL); and senior scholar guidance (JML, PAP).
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This project was partially supported through a research dissemination grant (VRI-D-2020-10-001-RDG) received from the Universidad Norbert Wiener by Dr. Patrick A. Palmieri to advance positive social change through promoting open-source knowledge for low- and middle-income countries.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank Dr. Mirella Brooks and Dr. Jonas Nguh for their contributions to the development of the protocol for this project.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers web site along with the published article.
REFERENCES
| [1] | Lyder CH, Ayello EA. Patient safety and quality: An evidence-based handbook for nurses 2008. |
| [2] | Jha A, Ed. Summary of the evidence on patient safety: Implications for research 2008. |
| [3] | Hahnel E, Lichterfeld A, Blume-Peytavi U, Kottner J. The epidemiology of skin conditions in the aged: A systematic review. J Tissue Viability 2017; 26(1): 20-8. |
| [4] | Al Mutairi KB, Hendrie D. Global incidence and prevalence of pressure injuries in public hospitals: A systematic review. Wound Medicine 2018; 22: 23-31. |
| [5] | Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: A systematic review. JAMA 2006; 296(8): 974-84. |
| [6] | Raetz JGM, Wick KH, Wick KW. Common questions about pressure ulcers. Am Fam Physician 2015; 92(10): 888-94. |
| [7] | Peterson MJ, Gravenstein N, Schwab WK, van Oostrom JH, Caruso LJ. Patient repositioning and pressure ulcer risk--monitoring interface pressures of at-risk patients. J Rehabil Res Dev 2013; 50(4): 477-88. |
| [8] | Lyder CH, Wang Y, Metersky M, et al. Hospital-acquired pressure ulcers: Results from the national Medicare Patient Safety Monitoring System study. J Am Geriatr Soc 2012; 60(9): 1603-8. |
| [9] | Hibbs P. The economics of pressure ulcer prevention. Decubitus 1988; 1(3): 32-8. |
| [10] | Bo M, Massaia M, Raspo S, et al. Predictive factors of in-hospital mortality in older patients admitted to a medical ICU. J Am Geriatr Soc 2003; 51(4): 529-33. |
| [11] | Khor HM, Tan J, Saedon NI, et al. Determinants of mortality among older adults with pressure ulcers. Arch Gerontol Geriatr 2014; 59(3): 536-41. |
| [12] | Paul M. Costs associated with pressure wounds in the U.S. inpatient hospital population. Innovation in Aging 2018; 2(suppl-1): 920-21. |
| [13] | Ackroyd-Stolarz S. Improving the prevention of pressure ulcers as a way to reduce health care expenditures. CMAJ 2014; 186(10): E370-1. |
| [14] | Meddings J, Reichert H, Rogers MAM, Hofer TP, McMahon LF Jr, Grazier KL. Under pressure: Financial effect of the hospital-acquired conditions initiative–A statewide analysis of pressure ulcer development and payment. J Am Geriatr Soc 2015; 63(7): 1407-12. |
| [15] | Young DL, Shen JJ, Estocado N, Landers MR. Financial impact of improved pressure ulcer staging in the acute hospital with use of a new tool, the NE1 Wound Assessment Tool. Adv Skin Wound Care 2012; 25(4): 158-66. |
| [16] | Padula WV, Makic MBF, Wald HL, et al. Hospital-acquired pressure ulcers at academic medical centers in the united states, 2008-2012: Tracking changes since the cms nonpayment policy. Jt Comm J Qual Patient Saf 2015; 41(6): 257-63. |
| [17] | Shahin ES, Dassen T, Halfens RJ. Pressure ulcer prevalence and incidence in intensive care patients: A literature review. Nurs Crit Care 2008; 13(2): 71-9. |
| [18] | VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008-2009 international pressure ulcer prevalence survey and a 3-year, acute care, unit-specific analysis. Ostomy Wound Manage 2009; 55(11): 39-45. |
| [19] | Compton F, Hoffmann F, Hortig T, et al. Pressure ulcer predictors in ICU patients: Nursing skin assessment versus objective parameters. J Wound Care 2008; 17(10): 417-20-22-4. |
| [20] | Chicano SG, Drolshagen C. Reducing hospital-acquired pressure ulcers. J Wound Ostomy Continence Nurs 2009; 36(1): 45-50. |
| [21] | Moore Z. Bridging the theory-practice gap in pressure ulcer prevention. Br J Nurs 2010; 19(15): S15-8. |
| [22] | Reilly EF, Karakousis GC, Schrag SPS. S. P. Pressure ulcers in the ICU: The ‘forgotten’ enemy. Scientist 2007; 1(2): 17-30. |
| [23] | Cox J. Predictors of pressure ulcers in adult critical care patients. Am J Crit Care 2011; 20(5): 364-75. |
| [24] | Heslop L, Lu S, Xu X. Nursing-sensitive indicators: A concept analysis. J Adv Nurs 2014; 70(11): 2469-82. |
| [25] | Elliott R, McKinley S, Fox V. Quality improvement program to reduce the prevalence of pressure ulcers in an ICU. Am J Crit Care 2008; 17(4): 328-34. |
| [26] | Reilly EF, Karakousis GC, Schrag SP, Stawicki SP. Pressure ulcers in the ICU: The ‘forgotten’ enemy. Scientist 2007; 1(2): 17-30. |
| [27] | Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: Systematic review. Int J Nurs Stud 2013; 50(7): 974-1003. |
| [28] | Black J, Berke C, Urzendowski G. Pressure ulcer incidence and progression in critically ill subjects: Influence of low air loss mattress versus a powered air pressure redistribution mattress. J Wound Ostomy Continence Nurs 2012; 39(3): 267-73. |
| [29] | Soban LM, Hempel S, Munjas BA, Miles J, Rubenstein LV. Preventing pressure ulcers in hospitals: A systematic review of nurse-focused quality improvement interventions. Jt Comm J Qual Patient Saf 2011; 37(6): 245-52. |
| [30] | Krupp AE, Monfre J. Pressure ulcers in the ICU patient: An update on prevention and treatment. Curr Infect Dis Rep 2015; 17(3): 468. |
| [31] | Joseph J, Clifton SD. Nurses' knowledge of pressure ulcer risk assessment. Nursing standard (Royal College of Nursing (Great Britain) : 1987) 2013; 27(33): 54, 56-8-60. |
| [32] | Tayyib N, Coyer F. Effectiveness of pressure ulcer prevention strategies for adult patients in ICUs: A systematic review. World views Evid Based Nurs 2016; 13(6): 432-44. |
| [33] | Niederhauser A, VanDeusen Lukas C, Parker V, Ayello EA, Zulkowski K, Berlowitz D. Comprehensive programs for preventing pressure ulcers: A review of the literature. Adv Skin Wound Care 2012; 25(4): 167-88. |
| [34] | Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Predicting pressure ulcer risk: A multisite study of the predictive validity of the Braden Scale. Nurs Res 1998; 47(5): 261-9. |
| [35] | Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Multi-site study of incidence of pressure ulcers and the relationship between risk level, demographic characteristics, diagnoses, and prescription of preventive interventions. J Am Geriatr Soc 1996; 44(1): 22-30. |
| [36] | Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nurs Res 1987; 36(4): 205-10. |
| [37] | Bergstrom N, Demuth PJ, Braden BJ. A clinical trial of the Braden Scale for predicting pressure sore risk. Nurs Clin North Am 1987; 22(2): 417-28. |
| [38] | Braden BJ. The Braden Scale for Predicting Pressure Sore Risk: Reflections after 25 years. Adv Skin Wound Care 2012; 25(2): 61. |
| [39] | Jochem K, Weigand L. Using a bundle approach to reduce pressure ulcers in an ICU. Am J Safe Patient Handl Mov 2014; 4(1): 15-20. |
| [40] | Tescher AN, Branda ME, Byrne TJO, Naessens JM. All at-risk patients are not created equal: Analysis of Braden pressure ulcer risk scores to identify specific risks. J Wound Ostomy Continence Nurs 2012; 39(3): 282-91. |
| [41] | Pancorbo-Hidalgo PL, Garcia-Fernandez FP, Lopez-Medina IM, Alvarez-Nieto C. Risk assessment scales for pressure ulcer prevention: A systematic review. J Adv Nurs 2006; 54(1): 94-110. |
| [42] | Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf 2005; 31(5): 243-8. |
| [43] | Institute for Healthcare Improvement. What is a bundle? 2017. Available at: http://www.ihi.org/resources/Pages/ImprovementStories/WhatIsaBundle.aspx |
| [44] | Robb E, Jarman B, Suntharalingam G, Higgens C, Tennant R, Elcock K. Using care bundles to reduce in-hospital mortality: Quantitative survey. BMJ 2010; 340: c1234. |
| [45] | Zuo X-L, Meng F-J. A care bundle for pressure ulcer treatment in ICUs. Int J Nurs Sci 2015; 2(4): 340-7. |
| [46] | Tricco AC, Ashoor HM, Cardoso R, et al. Sustainability of knowledge translation interventions in healthcare decision-making: A scoping review. Implement Sci 2016; 11(1): 55. |
| [47] | Curtis K, Fry M, Shaban RZ, Considine J. Translating research findings to clinical nursing practice. J Clin Nurs 2017; 26(5-6): 862-72. |
| [48] | Yost J, Ganann R, Thompson D, et al. The effectiveness of knowledge translation interventions for promoting evidence-informed decision-making among nurses in tertiary care: A systematic review and meta-analysis. Implement Sci 2015; 10(1): 98. |
| [49] | Keller BP, Wille J, van Ramshorst B, van der Werken C. Pressure ulcers in intensive care patients: A review of risks and prevention. Intensive Care Med 2002; 28(10): 1379-88. |
| [50] | Higgins JPT, Green S, Eds. Cochrane handbook for systematic reviews of interventions 2011. |
| [51] | Arksey H, O’Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol 2005; 8(1): 19-32. |
| [52] | Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med 2003; 96(3): 118-21. |
| [53] | Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid-Based Healthc 2015; 13(3): 141-6. |
| [54] | Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol 2011; 11(1): 15. |
| [55] | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 2009; 62(10): 1006-12. |
| [56] | Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 2009; 62(10): e1-e34. |
| [57] | Critical Appraisal Skills Programme. CASP cohort study checklist 2018. Available at: https://casp-uk.net/casp-tools-checklists/ |
| [58] | Critical Appraisal Skills Programme. CASP randomised controlled trial checklist 2018. Available at: https://casp-uk.net/casp-tools-checklists/ |
| [59] | Critical Appraisal Skills Programme. CASP systematic review checklist 2018.https://casp-uk.net/casp-tools-checklists/ |
| [60] | Armola RR, Bourgault AM, Halm MA, et al. Upgrading the American Association of Critical-Care Nurses’ evidence-leveling hierarchy. Am J Crit Care 2009; 18(5): 405-9. |
| [61] | Peterson MH, Barnason S, Donnelly B, et al. Choosing the best evidence to guide clinical practice: Application of AACN levels of evidence. Crit Care Nurse 2014; 34(2): 58-68. |
| [62] | Bergstrom N, Horn SD, Rapp MP, Stern A, Barrett R, Watkiss M. Turning for ulcer reduction: A multisite randomized clinical trial in nursing homes. J Am Geriatr Soc 2013; 61(10): 1705-13. |
| [63] | Chaboyer W, Bucknall T, Webster J, et al. The effect of a patient centred care bundle intervention on pressure ulcer incidence (INTACT): A cluster randomised trial. Int J Nurs Stud 2016; 64: 63-71. |
| [64] | Chou R, Dana T, Bougatsos C, et al. Pressure ulcer risk assessment and prevention: A systematic comparative effectiveness review. Ann Intern Med 2013; 159(1): 28-38. |
| [65] | Coladonato J, Smith A, Watson N, et al. Prospective, nonrandomized controlled trials to compare the effect of a silk-like fabric to standard hospital linens on the rate of hospital-acquired pressure ulcers. Ostomy Wound Manage 2012; 58(10): 14-31. |
| [66] | Cooper KL. Evidence-based prevention of pressure ulcers in the ICU. Crit Care Nurse 2013; 33(6): 57-66. |
| [67] | Cowan LJ, Stechmiller JK, Rowe M, Kairalla JA. Enhancing Braden pressure ulcer risk assessment in acutely ill adult veterans. Wound Repair Regen 2012; 20(2): 137-48. |
| [68] | Coyer F, Gardner A, Doubrovsky A, et al. Reducing pressure injuries in critically ill patients by using a patient skin integrity care bundle (InSPiRE). Am J Crit Care 2015; 24(3): 199-209. |
| [69] | Dutra RAA, Salomé GM, Alves JR, et al. Using transparent polyurethane film and hydrocolloid dressings to prevent pressure ulcers. J Wound Care 2015; 24(6): 268-275, 270-271, 273-275. |
| [70] | Edger M. Effect of a patient-repositioning device in an ICU on hospital-acquired pressure injury occurences and cost: A before-after study. J Wound Ostomy Continence Nurs 2017; 44(3): 236-40. |
| [71] | Estilo MEL, Angeles A, Perez T, Hernandez M, Valdez M. Pressure ulcers in the ICU: New perspectives on an old problem. Crit Care Nurse 2012; 32(3): 65-70. |
| [72] | Evans AM, Barklam D, Hone K, Ellis G. Reducing pressure damage: Care bundles and collaborative learning. British J Nursing 2013; 22(Sup12): S32-8. |
| [73] | Flike K. Pressure ulcer prevention in the ICU: A case study. Crit Care Nurs Q 2013; 36(4): 415-20. |
| [74] | Gillespie BM, Chaboyer WP, McInnes E, Kent B, Whitty JA, Thalib L. Repositioning for pressure ulcer prevention in adults. Cochrane Database Syst Rev 2014; 2014(4)CD009958 |
| [75] | Guihan M, Bombardier CH, Ehde DM, et al. Comparing multicomponent interventions to improve skin care behaviors and prevent recurrence in veterans hospitalized for severe pressure ulcers. Arch Phys Med Rehabil 2014; 95(7): 1246-1253.e3. |
| [76] | Hall KD, Clark RC. A prospective, descriptive, quality improvement study to investigate the impact of turn-and-position devices on incidence of hospital-acquired sacral pressure ulcers and nursing staff time needed for repositioning patients. Ostomy Wound Manage 2016; 62(11): 40-4. |
| [77] | Mallah Z, Nassar N, Kurdahi Badr L. The effectiveness of a pressure ulcer intervention program on the prevalence of hospital acquired pressure ulcers: Controlled before and after study. Appl Nurs Res 2015; 28(2): 106-13. |
| [78] | Meyers T. Prevention of heel pressure injuries and plantar flexion contractures with use of a heel protector in high-risk neurotrauma, medical, and surgical ICUs: A randomized controlled trial. J Wound Ostomy Continence Nurs 2017; 44(5): 429-33. |
| [79] | Ozyurek P, Yavuz M. Prevention of pressure ulcers in the ICU: A randomized trial of 2 viscoelastic foam support surfaces. Clin Nurse Spec 2015; 29(4): 210-7. |
| [80] | Park KH, Park J. The efficacy of a viscoelastic foam overlay on prevention of pressure injury in acutely ill patients: A prospective randomized controlled trial. J Wound Ostomy Continence Nurs 2017; 44(5): 440-4. |
| [81] | Ranzani OT, Simpson ES, Japiassú AM, Noritomi DT. The challenge of predicting pressure ulcers in critically ill patients. A multicenter cohort study. Ann Am Thorac Soc 2016; 13(10): 1775-83. |
| [82] | Smith A, McNichol LL, Amos MA, et al. A retrospective, nonrandomized, beforeand- after study of the effect of linens constructed of synthetic silk-like fabric on pressure ulcer incidence. Ostomy Wound Manage 2013; 59(4): 28-30, 32-34. |
| [83] | Tayyib N, Coyer F, Lewis PA. Implementing a pressure ulcer prevention bundle in an adult intensive care. Intensive Crit Care Nurs 2016; 37: 27-36. |
| [84] | Tescher AN, Thompson SL, McCormack HE, et al. A retrospective, descriptive analysis of hospital-acquired deep tissue injuries. Ostomy Wound Manage 2018; 64(11): 30-41. |
| [85] | Thorpe E. Prophylactic use of dressings for pressure ulcer prevention in the critical care unit. Br J Nurs 2016; 25(12): S6-S12. |
| [86] | Twersky J, Montgomery T, Sloane R, et al. A randomized, controlled study to assess the effect of silk-like textiles and high-absorbency adult incontinence briefs on pressure ulcer prevention. Ostomy Wound Manage 2012; 58(12): 18-24. |
| [87] | Yap TL, Kennerly SM. A nurse-led approach to preventing pressure ulcers. Rehabil Nurs 2011; 36(3): 106-10. |
| [88] | Yap TL, Kennerly SM, Bergstrom N, Hudak SL, Horn SD. An evidence-based cue-selection guide and logic model to improve pressure ulcer prevention in long-term care. J Nurs Care Qual 2016; 31(1): 75-83. |
| [89] | Webster J, Coleman K, Mudge A, et al. Pressure ulcers: effectiveness of risk-assessment tools. A randomised controlled trial (the ULCER trial). BMJ Qual Saf 2011; 20(4): 297-306. |
| [90] | Anderson M, Finch Guthrie P, Kraft W, Reicks P, Skay C, Beal AL. Universal pressure ulcer prevention bundle with WOC nurse support. J Wound Ostomy Continence Nurs 2015; 42(3): 217-25. |
| [91] | Armour-Burton T, Fields W, Outlaw L, Deleon E. The Healthy Skin Project: Changing nursing practice to prevent and treat hospital-acquired pressure ulcers. Crit Care Nurse 2013; 33(3): 32-9. |
| [92] | Cano A, Anglade D, Stamp H, et al. Improving outcomes by implementing a pressure ulcer prevention program (PUPP): Going beyond the basics. Healthcare (Basel) 2015; 3(3): 574-85. |
| [93] | Kelleher AD, Moorer A, Makic MF. Peer-to-peer nursing rounds and hospital-acquired pressure ulcer prevalence in a surgical ICU: A quality improvement project. J Wound Ostomy Continence Nurs 2012; 39(2): 152-7. |
| [94] | Padula WV, Gibbons RD, Valuck RJ, et al. Are evidence-based practices associated with effective prevention of hospital-acquired pressure ulcers in US academic medical centers? Med Care 2016; 54(5): 512-8. |
| [95] | Qaseem A, Mir TP, Starkey M, Denberg TD. Risk assessment and prevention of pressure ulcers: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2015; 162(5): 359-69. |
| [96] | Sullivan N. Preventing In-Facility Pressure Ulcers as a Patient Safety Strategy. Annals of Internal Medicine 2013; 158(5_Part_2): 410-16. |
| [97] | Swafford K, Culpepper R, Dunn C. Use of a comprehensive program to reduce the incidence of hospital-acquired pressure ulcers in an ICU. Am J Crit Care 2016; 25(2): 152-5. |
| [98] | Tayyib N, Coyer F, Lewis PA. A two-arm cluster randomized control trial to determine the effectiveness of a pressure ulcer prevention bundle for critically ill patients. J Nurs Scholarsh 2015; 47(3): 237-47. |
| [99] | Becker D, Tozo TC, Batista SS, et al. Pressure ulcers in ICU patients: Incidence and clinical and epidemiological features: A multicenter study in southern Brazil. Intensive Crit Care Nurs 2017; 42: 55-61. |
| [100] | Coyer F, Miles S, Gosley S, et al. Pressure injury prevalence in intensive care versus non-intensive care patients: A state-wide comparison. Aust Crit Care 2017; 30(5): 244-50. |
| [101] | Gray-Siracusa K, Schrier L. Use of an intervention bundle to eliminate pressure ulcers in critical care. J Nurs Care Qual 2011; 26(3): 216-25. |
| [102] | O’Brien G, Moore Z, Patton D, O’Connor T. The relationship between nurses assessment of early pressure ulcer damage and sub epidermal moisture measurement: A prospective explorative study. J Tissue Viability 2018; 27(4): 232-7. |
| [103] | Moya-Suárez AB, Canca-Sánchez JC, Enríquez de Luna-Rodríguez M, Aranda-Gallardo M, Morales-Asencio JM. Factors associated with variability in the prevention of pressure ulcers. J Tissue Viability 2018; 27(4): 211-6. |
| [104] | Kottner J, Cuddigan J, Carville K, et al. Pressure ulcer/injury classification today: An international perspective. J Tissue Viability 2020; 29(3): 197-203. |
| [105] | Barakat-Johnson M, Lai M, Barnett C, et al. Hospital-acquired pressure injuries: Are they accurately reported? A prospective descriptive study in a large tertiary hospital in Australia. J Tissue Viability 2018; 27(4): 203-10. |
| [106] | Ham WHW, Schoonhoven L, Schuurmans MJ, Veugelers R, Leenen LPH. Pressure ulcer education improves interrater reliability, identification, and classification skills by emergency nurses and physicians. J Emerg Nurs 2015; 41(1): 43-51. |
| [107] | Lahmann NA, Kottner J. Relation between pressure, friction and pressure ulcer categories: A secondary data analysis of hospital patients using CHAID methods. Int J Nurs Stud 2011; 48(12): 1487-94. |
| [108] | Garcia-Fernandez FP, Pancorbo-Hidalgo PL, Agreda JS, Torres MR. Risk assessment scales for pressure ulcers in ICUs: A systematic review with meta-analysis. European Wound Management Association Journal 2013; 13(2): 7-13. |
| [109] | Sebastián-Viana T, Losa-Iglesias M, González-Ruiz JM, Lema-Lorenzo I, Núñez-Crespo FJ, Salvadores Fuentes P. Reduction in the incidence of pressure ulcers upon implementation of a reminder system for health-care providers. Appl Nurs Res 2016; 29: 107-12. |
| [110] | Raju D, Su X, Patrician PA, Loan LA, McCarthy MS. Exploring factors associated with pressure ulcers: A data mining approach. Int J Nurs Stud 2015; 52(1): 102-11. |
| [111] | Demarré L, Van Lancker A, Van Hecke A, et al. The cost of prevention and treatment of pressure ulcers: A systematic review. Int J Nurs Stud 2015; 52(11): 1754-74. |
| [112] | Coleman S, Smith IL, Nixon J, Wilson L, Brown S. Pressure ulcer and wounds reporting in NHS hospitals in England part 2: Survey of monitoring systems. J Tissue Viability 2016; 25(1): 16-25. |









