All published articles of this journal are available on ScienceDirect.
What is the Current Effectiveness of Olaparib for Breast Cancer Patients with a BRCA Mutation? A Systematic Review
Abstract
Background:
The Poly (ADP-ribose) polymerase inhibitor olaparib, acts against cancer cells in people with breast cancer pre-disposition gene mutations (BRCAm). Despite US and EU approval as a therapy for ovarian cancer patients with BRCAm, but research into olaparib therapy for breast cancer patients with BRCAm is in its infancy.
Objective:
As no systematic review has yet been undertaken to synthesise clinical trials looking at olaparib as a therapy for breast cancer patients with BRCAm, this systematic review aims to establish the current effectiveness of olaparib as a treatment for these patients.
Methods:
CINAHL, MEDLINE, Royal College of Nursing, Cochrane Library, Joanna Briggs Institute, Centre for Reviews and Dissemination, Internurse, Embase, Google Scholar and PubMed databases were searched, supplemented by a grey literature search, hand searching and cross-referencing. Authors independently reviewed and graded the studies also using Kmet et al. scoring system.
Results:
One long-term case study and six clinical trials were included. Heterogeneity prevented statistical meta-analysis, meaning only narrative synthesis was possible. The overall clinical benefit of olaparib appears to be greater and longer lived in BRCAm carriers compared to BRCAwt, and also when compared to standard chemotherapy treatments.
Conclusion:
Implications for nursing: nurses working in this field should be aware that the most compelling results were found in the subset of patients who harbour a BRCA mutation, meaning that olaparib should be regarded as a clinically effective potential therapy for these patients. Larger, longer-term trials including comparator arms are required to demonstrate benefits including overall survival, adverse effects and quality of life.
1. INTRODUCTION
Alterations in the breast cancer (BC) genes, BRCA1 and BRCA2, can lead to autosomal dominant, highly penetrant, predisposition to breast and ovarian cancers, with lifetime risks as high as 84% [1].
BRCA-mutant (BRCAm) breast cancers are more often high-grade Triple Negative Breast Cancer (TNBC) which means they are negative for oestrogen receptor, progesterone receptor, and human epidermal growth factor receptor2 (HER2), making them much harder to target therapeutically [2]. TNBC has the poorest overall survival of all breast cancer subtypes with the highest rates of metastatic disease [2]. Recent studies have revealed an emerging therapy using Poly (ADP-ribose) polymerase inhibitors (PARPi), which has had great success when treating BRCAm ovarian cancers. Olaparib is one of several known PARPis and has been in trials as a monotherapy, and in combination other standard therapies, and as maintenance therapy [3-6]
Olaparib has been granted approval in the USA, and in the EU to be used as a therapy for Ovarian Cancer (OC) patients with BRCAm, but as yet the research into olaparib therapy for BC patients with BRCAm is in its infancy.
No Systematic Review (SR) has been undertaken to synthesisethe clinical trials looking at olaparib as a therapy for BC patients with BRCAm. Given olaparib’s success with BRCAm OC, there is a clear need for an SR and synthesis of the trials investigating olaparib as a therapy in BRCAm BC patients. This paper is the first to conduct such an SR and draw tentative conclusions and recommendations based on a narrative review of the evidence.
In addition to germline BRCA tumours, BRCA deficiency is observed in somatic BRCAm tumours [7], so this SR will look at all BRCAm patients regardless of whether the BRCAm was germline or somatic.
Firstly, we will outline the basic science and clinical applications of BRCAm BC, PARPi, and olaparib.
1.1. Aetiology of BRCAm Breast Cancer
Mistakes (mutations) in DNA replication and failures of DNA repair pathways are central to the development of cancer [8]. Cancer cells are cells that have acquired malignant properties such as proliferation, invasion, and metastasis. These malignant cells can evade apoptosis (controlled cell death). Many chemotherapies and some targeted agents work by creating catastrophic damage to DNA in malignant cells, as to make the cell non viable [8].
BRCA1 and BRCA2 are genes involved in DNA repair - they are broadly categorised as tumour suppressors [9]. So if this repair pathway is impaired (e.g. by BRCAm), then these patients are at increased risk of developing BC, OC and other cancers [10].
1.2. Synthetic Lethality
DNA continually sustains damaging mutations under a barrage of environmental and lifestyle assaults like UV light, tobacco, toxic products of metabolism, all promoting faulty DNA replication [8]. Various DNA repair mechanisms have evolved to repair these mutations to maintain genomic integrity. A predominant repair pathway utilises Poly (ADP-ribose) polymerase (PARP) enzymes [9].
BRCAm cancer cells have lost both copies of their normal BRCA gene, so they lose normal BRCA repair activity, whereas non-tumour cells maintain one functional copy of the BRCA gene [8].
This deficiency in repair makes BRCAm cancers hypersensitive to DNA damaging treatments such as platinum chemotherapy [9] and Poly (ADP-ribose) polymerase inhibitors [PARPi] [11, 12]. With PARP inhibition (PARPi), DNA cannot be repaired in BRCAm cancer cells [13].
Cells with a working BRCA gene can repair any damage to the double helix. Cells without a functioning BRCA gene rely on PARP to repair the damage, so a deficiency of one repair pathway alone (PARP or BRCA) has no impact on cell viability. However, if PARP is inhibited in addition to BRCA being mutated, then the loss of both BRCA and PARP repair pathways results in cell death. This is a concept called synthetic lethality and leads to cell cycle arrest and apoptosis (controlled cell death) in these cancer cells, i.e. PARPi induces synthetic lethality in BRCAm tissues [9, 13]. Olaparib is one of the first known PARPi to exploit this synthetic lethality.
1.3. Current Status of Parp Inhibitors
PARP inhibitors (PARPi) have been the centre of a great deal of new research into potential new anti-tumour agents since the concept of synthetic lethality was introduced, when BRCAm tumour cells showed over 1000 fold greater sensitivity to PARPi in preclinical models [11, 12]. Since then, a great deal of research has been done looking at olaparib as a therapy for OC patients with BRCAm [3, 4, and 5]. Clinical studies have verified anti-tumour activities of PARPi in BRCA mutant OC tumours [3] and in contrast to the limited efficacy of PARPi alone, the combination of PARPi with DNA repair defects is often lethal to tumour cells. The clinical efficacy of PARPi as single agents has shown only moderate efficacy, except for in BRCAm or BRCA-like tumours [14].
In December 2014, twenty years after the discovery of the BRCA genes, olaparib (the first in human PARPi) was approved for treatment of patients with germline BRCA associated OC with three or more prior lines of chemotherapy [15]. This approval represents the first ‘personalised’ therapy for OC. As well as the approval of olaparib was the approval of the Myriad Genetics BRCAnalysis CDz TM test as the BRCAm diagnostic test to identify patients eligible for olparib treatment.
Comparatively, olaparib as a therapy for BC patients with the same BRCA deficiency is lagging behind with only one phase 3 trial completed to date, and another ongoing.
1.4. Olaparib/Lynparza
Although olaparib therapy has been approved for OC in the USA and the EU, the optimal application of olaparib in the treatment of BRCAm BC has not yet been determined.
In 2012, Ledermann et al. [5] published the results of his phase 2 trial on olaparib as maintenance therapy for platinum-sensitive OC patients. They reported that maintenance olaparib significantly improved progression-free survival (PFS) in a randomized, double-blind, placebo-controlled trial. Of note - there was a subset of patients (11%) in Ledermann et al. study [5], including patients with BRCAm and BRCAwt whose results showed long-term disease control on maintenance olaparib, and progression-free survival (PFS) of over 5 years.
Despite this, the drug failed to induce prolonged overall survival (OS) - 34.9 months versus 31.9 months. As a result of the benefit of PFS not translating into an improvement in OS, the sponsor announced it would abandon plans into phase 3 trials of olaparib therapy, so further development was put on hold.
A year later, the results from Ledermann et al. [5] pre-planned analysis of the data based on BRCAm status was presented at the 2013 ACSO annual meeting. Out of 131 patients receiving olaparib (plus 123 patients receiving placebo), 56% and 50% of patients had BRCAm or suspected BRCAm. The pre-planned retrospective analysis (done after patients were reassigned by BRCA status after confirmed Myriad testing), revealed that PFS was significantly longer in BRCAm patients than BRCA wild-type (wt) patients (11.2 months versus 4.3 months). These results demonstrated that OC patients with BRCAm might benefit from olaparib therapy - and also indicated that olaparib monotherapy was effective against platinum-sensitive recurrent OC with BRCAm [5]
This positive result led by Astra Zeneca (AZ) to reverse their decision in 2013 and restart the phase 3 clinical trials of olaparib. Additionally, in response to these results, the European Medicines Agency (EMA) also approved olaparib as a maintenance therapy on 18th December 2014 for platinum-sensitive OC in patients with BRCAm. The clinical data from these trials led to olaparib receiving approval by the US food and drug administration (FDA) in 2014 for the fourth line or maintenance treatment of BRCAm OC [15].
The approval of olaparib marked PARPi as a success and reactivated research into PARPi inhibitors as a therapeutic strategy for all cancers, especially those with BRCAm. Since that time, there have been two key lines of investigation into the concept of PARPi as a treatment - PARPi monotherapy and PARPi combined with DNA-damaging chemotherapy.
The USA granted approval for olaparib monotherapy to be used in patients with germline (g) BRCAm or suspected gBRCAm (as detected by Myriad’s FDA-approved test) advanced OC treated with three or more prior lines of chemotherapy [16]. At the 2017 American Society of Clinical Oncology (ASCO) annual meeting, in discussion regarding metastatic BC patients harbouring BRCAm, olaparib was shown to be superior to conventional chemotherapy - defining a potential novel treatment standard in this high-risk population.
1.5. Rationale for this Systematic Review
Olaparib is an emerging treatment with some questions about its efficacy as a treatment for BRCAm used singly or in combination. A Systematic Review (SR) is necessary to evaluate, compare and contrast scientific studies which examine combinations of therapies, dosage regimes, and outcomes including survival, remission or otherwise in the existing primary studies.
1.6. Aims and Objectives
This systematic review aims to investigate the current effectiveness of olaparib in patients with BC and a BRCA mutation, and to produce and present this information in such a way as to reduce bias or inaccuracies. This is fundamental to the principle of SR synthesis. This review also aims to make clear links between the studies and the conclusions and identify any controversies, weaknesses and gaps in the field.
2. METHODS
The methods for this SR were made explicit in a protocol (written in accordance with PRISMA-P guidelines), and published on PROSPERO (CRD42018087832), which is an international database of prospective SRs recording the key features from a review protocol.
PRISMA focuses on the reporting of reviews evaluating RCTs and other types of research [particularly evaluations of interventions] [17]. The Preferred Reporting Items for Systematic reviews and Meta-Analyses for Protocols 2015 [18] is a reporting guideline for protocols, consisting of a 17-item checklist intended to facilitate the preparation and reporting of a robust protocol for the SR [19].
2.1. Review Question
Key questions are commonly formulated according to the ‘PICO’ method, which defines the population (P), the intervention (I), the control/comparator (C), and the outcome (O) [20]. Describing the criteria for each of the PICO elements enables the researcher to understand what is relevant, and what is not relevant, to the specific question being asked [21]. The population for studies in this review is patients over the age of 18 with BC and BRCAm. Gender is not a variable in this study, although it is a fact that BC typically affects more women than men. The intervention is olaparib, in any dosage, either as a monotherapy or in combination with other therapies. The best trials will be phase 3 double-blind randomised clinical trials and have a placebo arm, or comparator intervention(s). However given the infancy of the research into olaparib in BC patients, most studies will be phase 1 or 2, and some studies will be a single arm. Overall Survival (OS) and longevity of Progression-Free Survival (PFS) are the ideal endpoints for patients. However, RCTs measure how the intervention works, so the Objective Response Rate (ORR) - Complete Response (CR), Partial Response (PR) or no response - will be the primary endpoint. Adverse Events (AE) are also measured and discussed. Analysis of the pharmacokinetic responses or any other focus isbeyond the scope of this SR.
Based on this PICO, the literature search question is ‘What is the Current Effectiveness of Olaparib for Patients with Breast Cancer and a BRCA Mutation?’
2.2. Search Strategy
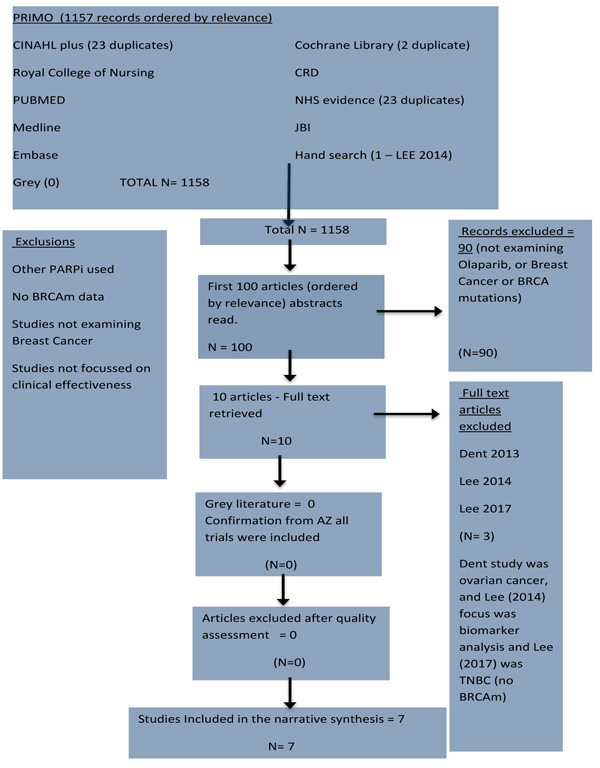
Fig. (1) shows the methodical approach based on PRISMA guidelines for undertaking reviews using electronic databases to search the literature (supplemented by hand searching and cross-referencing). A literature search of databases was completed in January 2018. First, there is an initial database search using keywords - and identification of potentially relevant literature from all databases, as well as from grey literature. After that, the literature is screened using the inclusion and exclusion criteria, followed by a decision about its quality, followed by a final decision to include that literature or not, with reasons why. The resulting evidence should meet all the criteria to be included in the synthesis of the literature [21].

Initial searches on PRIMO were based on a predetermined series of keywords as follows: Olaparib/ Lynparza, Breast Cancer, and BRCA mutation (including all permutations). PRIMO database searches 179 databases including 43 databases under Nursing and Medicine which include Core databases such as CINAHL, MEDLINE, Royal College of Nursing (RCN), Cochrane Library, Joanna Briggs Institute (JBI), CRD, Internurse, Embase, Google Scholar, PubMed. Searches of specialist databases (Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane library, Joanna Briggs Institute (JBI), National Health Service (NHS) website, and Clinical trials UK). Hand-searching and cross-referencing werealso undertaken. The first author contacted authors of several unpublished studies, as well as the UK’s leading expert on olaparib in BC patients with BRCAm (Professor James Tutt), and also the pharmaceutical company that manufactures olaparib to discuss trials they are involved in, their transparency biomedical policy and ethical code of conduct. The date for inclusion of papers in this systematic review is 2009, which is when Fong et al. (2009) key paper on maximum tolerated dose (MTD) of olaparib was published [22].
After the literature search was undertaken, preliminary scanned or material to get a general sense of their contents and abstracts, next, inclusion and exclusion criteria were applied, these were: English language papers on BRCAm BC treated with olaparib, in scientific professional peer reviewed journals and conference proceedings, focussing on clinical effect were included. Studies not focussed on breast cancer data, or BRCAm data, or PARPi other than olaparib were excluded. Pre-clinical studies were not included. Studies whose focus lies outside of clinical effectiveness were also not included.
2.3. Quality Assessment and Eligibility for Inclusion
Kmet et al. [23] was used for quality assessment, carried out independently by two independent assessors (the two authors) and the papers graded. Kmet et al. [23] contains a 14-point checklist for assessing the quality of quantitative studies with a scoring system for each category and whether the study meets that criteria or not (2 points for yes, 1 point for partial, and 0 points for no) [23]. For this SR, the authors agreed ona cut-off score for inclusion of atleast 0,75 or 75%. All the studies eventually included in the review scored over 75%.
2.4. Ethical Issues
As this is secondary research there is no need for ethical approval, however, the primary research should all be approved by review boards and ethics committees for each trial centre.
3. RESULTS
3.1. Meta-Analysis and Heterogeneity
The quantitative synthesis of results obtained from different studies is termed meta-analysis [24]. Meta-analysis can facilitate the synthesis of a large number of studies, and allow interpretation of studies with different sizes and estimates. As meta-analysis considers more than a single study, there is less uncertainty and results can facilitate evidence-based decision making in health care [17]. By collating information from different studies, meta-analysis can detect associations that a single study may not be able to provide.
The aim of meta-analysis is to estimate an overall effect and whether the effect is similar or dissimilar. If studies are relatively homogeneous they can be combined statistically and outcomes can be analysed and pooled. Meta analysis is usually presented as the extent to which a change based on an intervention was affected and then presented in a forest plot [21]. Meta analysis is a mathematical synthesis of the results of two or more primary studies that addressed a similar hypothesis [25].
Deciding when a meta-analysis is appropriate can be difficult because there are several sources of heterogeneity to be considered. If the designs, methods, quality, and results of the studies are deemed very heterogeneous, then statistically combining them can result in misleading conclusions. So a meta-analysis, in this case, would be inappropriate as it would be like attempting to ‘combine apples and pears’ [17].
It is important to consider the key clinical and methodological differences between the qualifying studies in order to assess their heterogeneity. Clinical differences include population - geography (UK, EU or global) and intervention (dosages) [17]. Methodological differences would include study design (cohort, case study, experimental design), reported outcomes (endpoints such as ORR, OS, PFS), and timing of outcomes [17].
As none of the studies in this SR address the same hypothesis in the same way, and have both clinical and methodological differences, a meta-analysis would not be appropriate, in which case, CRD [26] suggests that a narrative synthesis of studies, rather than statistical analysis, may be undertaken where studies are too heterogeneous (either clinically or methodologically) to combine. This approach clarified the similarities and differences among studies that appear to address the same or similar research questions [26].
Fig. (2) details the results of the search strategy in a PRISMA diagram and shows that 1157 records were found on PRIMO and ordered by relevance. The top 100 records were screened. Ninety studies were rejected and ten full texts were retrieved and read. Three records were rejected. All articles passed the quality assessment for inclusion. Seven studies met and passed all the criteria for inclusion in quantitative synthesis. All seven met the minimum Kmet et al. threshold score of 75%.
Table 1 shows the papers that were included in the review.
In addition to searching on PRIMO, separate searches were conducted on Cumulative Index to Nursing and Allied Health Literature (CINAHL) (23 records found but none additional), and Cochrane Library (2 records found (not additional) plus several abstracts), Centre for Reviews and Dissemination (CRD) (no records found) and Joanna Briggs Institute (JBI) (no records found), National Health Service (NHS) Evidence (23 records found but none additional), and National Institute for Health and Care Excellence (NICE) (2 records found, but none additional).
Hand searching and cross-referencing found no additional studies, while agrey literature search found no studies, but it did provide some current information from Professor James Tutt, one of the leading researchers in this field.
| Authors | Study Design | Methods of Data Collection and Analysis | Objective ResponseResults in BRCAm cohorts | Olaparib Dose | No. of Patients(No. with BRCAm BC) | Comments |
|---|---|---|---|---|---|---|
| Fong, 2009 | Phase 1 non randomised open label dose finding trial using 3+3 design.Single armmonotherapy | ORR, CR, PR measured using RECIST and CT or MRI scan. AEs measured using CTCAE | 33% at 400mg BiD | 10-600 BID | 60 (3) | Very small cohort. No written consent found. First in human trial. MTD found at 400 BiD |
| Tutt, 2010 | Phase 2 non randomised proof of concept open labelmonotherapy | ORR,CR, PR measured using RECIST and CT or MRI scan, AEs measured using CTCAE | 41% at 400mg BiD22% at 100 BiD | 400 or 100 BID | 54 (54) | Comparator arm on lower dose (100mg) = MTD is most effective. |
| Gelmon, 2011 | Phase 2 non randomisedOpen labelFour cancers studied. monotherapy | ORR,CR, PR measured using RECIST and CT or MRI scan, AEs measured using CTCAE | 0% | 400 BID | 90 (10) | Compares results between BRCAm or BRCAwt. Very small cohort. Heavily treated - 70% > 3 prior chemotherapy,TNBC = large heterogeneity among patients.3 patients data not confirmed. |
| Balmana, 2014 | Phase 1 non randomised dose finding trial, 3+ 3 design.Open-labelCombination therapy (with cisplatin) | ORR,CR, PR measured using RECIST and CT or MRI scan, AEs measured using CTCAE | 71%SD (>1 year) occurred in 5 breast cancer patients | 50-200 BID (continuous and intermittent dosing schedules) | 53 (17) | Patients BRCA status not centrally validatedSmall cohort. cisplatin 60 mg/m2 with intermittent olaparib 50 mg BID deemed tolerable but MTD not reached |
| Van der Noll, 2015 | Open-label Monotherapy long-term safety study following a Phase 1 single arm combination study (olaparib with carboplatin and/or paclitaxel). | AEs measured using CTCAE | N/A | 400BiD | 21 (5) | No written consent found. AEs reduced over time suggesting carryover from prior chemotherapy study. Mistake found in results text. Patients on therapy for up to 3.5 years. |
| Kaufman, 2015 | Phase 2 non randomisedOpen-label single armmonotherapy | ORR,CR, PR measured using RECIST and CT or MRI scan, AEs measured using CTCAE | 12.9% | 400 BID | 317 (62) | Very heavily pre-treated (average 4.6 prior therapies). No written consent found. 47% SD is a very good response rate given the heavy pre-treatment of these patients. |
| Robson, 2017 | Phase 3 randomisedOpen labelmonotherapy | ORR,CR, PR measured using RECIST and CT or MRI scan, AEs measured using CTCAE | 59.9% | 300 BID (tablet form) | 302 (302) | Not truly randomised as physicians choice was limited - and no placebo arm. Least pre-treated cohort with some patients only one prior treatment. Also measured QoL and PFS2. End point PFS was reached. |
| Study Reference | Study Design | Single Arm or Comparator | Combination or Mon Therapy? | Country or International | Clinical Trial Number | Ethical Approval |
|---|---|---|---|---|---|---|
| Fong, 2009 | Phase 1non-randomiseddose finding trial | Single arm | monotherapy | UK (Royal Marsden) and Netherlands (Cancer Institute) | NCT00516373 | Non mentioned |
| Tutt, 2010 | Phase 2Non-randomisedProof of conceptSequential cohort | Comparator arm on lower dose (100mg) | Monotherapy | Multinational - 26 centres across Australia, Germany, Spain, Sweden, UK and USA. | NCT00494234 | Written informed consentStudy approved by independent ethics committee for each trial centres, done in accordance with good clinical practice guidelines and Declaration of Helsinki |
| Gelmon, 2011 | Phase 2Non-randomisedOpen-label | Single arm but can compares results between BRCAm or BRCAwt | monotherapy | 6 centres across Canada. | NCT00679783 | Written informed consent. Study protocol approved by health Canada and institutional review boards and the six particiapating sites. |
| Balmana, 2014 | Phase 1`Non-randomisedDose finding trial | Single arm | Combination therapy (olaparib with cisplatin) | Multi centre - country not stated but Balmana works out of University Hospital in Barcelona. | NCT00782574 | Study carried out in accordance with declaration of Helsinki, good clinical practice and AZ policy on bioethics. |
| Kaufman, 2015 | Phase 2Non-randomised | Single arm but can compare different cancer types (ovarian, breast, pancreas, prostate and other) | monotherapy | Multinational - 13 centres across Israel, Australia, Germany, Spain, Sweden and USA | NCT01078662 | Written informed consent.Study carried out in accordance with international conference on harmonisation good clinical practice guidelines and declaration of Helsinki and approved by an independent ethics committee or institutional review board at every trial centre. |
| Van der Noll, 2015 | Long-term monotherapy safety study, following a phase 1 combination study | Single arm but can compare different cancer types (breast, ovarian and fallopian tube). | Monotherapy long-term safety study following a Phase 1 combination study (olaparib with carboplatin and/or paclitaxel). | Netherlands Cancer Institute | n/a | No mention of ethical approval |
| Robson, 2017 | Phase 3RandomisedOpen-label | Comparator arm was standard single chemotherapy of physicians choice (capecitabine, eribulin, or vinorelbine) | monotherapy | International - multi centres across 19 countries (Bulgaria, China, Czech Republic, France, Hungary, Italy, Japan, Korea, Mexico, Peru, Poland, Romania, Russia, Spain, Switzerland, Taiwan, Turkey, UK and USA.. | NCT02000622 | Protocol approved by ethics written informed consent. review committees at participating institutions |
To elaborate on grey literature: an hour-long conversation was held with the Medical Officer at AZ (who manufacture olaparib), who confirmed that, in his opinion, all the existing studies are included in this SR. He also confirmed that in OlympiA (NCT02032823), which is an ongoing phase 3 RCT sponsored by AZ, results were not available yet (originally due out in summer 2018 and now not expected until summer 2020) due to patient survival. The end point was a set number of AEs, which has not been reached at this time of writing. Neo-Olympia is another phase 3 trial looking at olaparib monotherapy in patients pre and post surgery, but the trial is ongoing and there are no results available yet. OlympiA, neo Olympia and OlympiAD are the only phase 3 trials to date looking at the effectiveness of olaparib in BC patients with BRCAm.
Professor Balmana was also contacted to ask about her recent research ‘Phase I, Open-Label, 2 Part Multicentre Study to Assess the Safety and Efficacy of Olaparib in Combination With Carboplatin in Patients With Advanced HER-2 Negative Breast Cancer’ (NCT02561832) and she replied to say that the trial stopped recruitment early and there is no efficacy data. The authors did not receive a reply about another ongoing trial by Abraham et al., although it appearslikely that this study is only just be starting. This is a randomised phase 2/3 trial to evaluate the safety and efficacy of the addition of olaparib to platinum-based neoadjuvant chemotherapy in triple negative and/or germline BRCAm breast cancer patients [27].
3.1.1. Detailed Narrative Review and Critical Appraisal
Six of the trials in this review are experimental, while one [28] is a long-term case study. All seven clinical trials in this SR vary in methods used. As shown in Table 1, all but Tutt et al. [29] and Robson et al. [30] are single arm studies. The interventions are different Fong et al. [22] and Balmana et al. [31] are dose-finding trials with increasing doses of olaparib. Fong et al. [22], Tutt et al. [29], Gelmon et al. [32], Kaufmann et al. [33], Van der Noll et al. [28] and Robson et al. [30] study olaparib monotherapy at MTD while Balmana et al. [31] investigate a combination therapy of olaparib with platinum treatment. None of the trials are randomised except Robson et al. [30] where patients were randomised to olaparib therapy or one of three therapies of physician’s choice. Table 2 shows the methodologies of the included studies.
The Consolidated Standards of Reporting Trials (CONSORT) is a checklist and flow diagram that was developed to improve the transparency and quality of reporting of RCTs, and to improve reliability and validity of trial findings [19]. All trials in this SR were found to abide by the CONSORT checklist [34]. However, none of the trials (except Robson et al. [30]) in this SR used randomisation or blinding so those sections of the CONSORT checklist and diagram were not reported (except Robson et al. [30]). The CONSORT 2010 Statement and website (www.consort-statement.org) also helps authors to critically appraise and interpret RCTs, and extract information for SRs, and the CONSORT checklist 2010 was used for this purpose
RCTs or true experiments are the most robust design for testing cause and effect relationships (e.g., whether a treatment or intervention affects outcomes) [35]. An RCT is an experiment with a random allocation of participants between experimental and control groups. The outcomes of the groups can then be compared [36]. For an RCT to be a true experiment, the following features apply: comparison and placebo arms, sampling and demographic equality at baseline, sample size estimation and power calculation, blinding and randomisation, consideration of drop-out rates and patient loss, and ethics and consent [21]. How these features appear in the studies within this SR is discussed below. RCTs in this SR were also examined for variation in study endpoints and data collection and analysis methods in order to collect all the evidence [37].
3.1.2. Comparison and Placebo Arms
Comparator arms are important in order to be sure that the effects noted are due to the actions of the intervention, and not due to anything else [17, 38]. Variables are manipulated and outcomes assessed between the experimental and control groups, so bias and other confounders can be controlled and factored out, and external influences removed, so that researchers can be sure effects noted are due to actions of the intervention and nothing else [17]. However only Robson et al. [30] study had true comparator arms with patients taking different interventions (although Van der Noll et al. [28] and Kaufman et al. [33] could compare results between different cancers, while Tutt et al. [29] could compare different dosages and Gelmon et al. [32] could compare BRCAm with BRCAwt results.
For trials like the ones in this SR that are not placebo-controlled, there is no comparator so results may be misleading or biased, especially when it comes to reporting the efficacy of treatments [39]. Placebo arms are required to test a hypothesis, but being as there were no placebo arms in any RCTs, this reduces the external validity of results of all the studies and is a serious limitation to this SR. Additional weaknesses to Robson et al. [30] trial are highlighted by a lack of a platinum-based chemotherapy comparator arm, which seems strange given the evidence of platinum sensitivity correlating to olaparib sensitivity noted in previous studies [3-5]. RCTs with comparator arms and large sample sizes provide the most reliable evidence regarding the efficacy of healthcare interventions [36], however, this is not the case with the seven studies considered as part of this SR.
3.1.3. Sampling and Demographic Equality of Groups at Baseline
It is not possible to include all potentially eligible subjects of a population so samples are taken and these should be random [21]. Data from randomly selected samples are generalisable to the target population and sometimes beyond (too similar populations and settings) [17]. However sampling for the trials within this SR has been dictated by a specific characteristic in a population (BC with BRCAm), so sample sizes are often small, not randomly selected and results are relevant only for this subset of patients. Sacket et al. [40] remind us however that evidence-based medicine is not restricted to randomised RCTs and that to find out about a specific therapy for a specific group of patients, then we need studies using those patients who harbour the relevant disorder (in this case BC with BRCAm). Selection bias in these RCTs is not a factor, as selection bias only occurs when the participants are not a true representative sample of the target population about whom the conclusions will be drawn [41].
Baseline demographic characteristics of patients were all similar in these studies, and are balanced between the treatment groups (age, gender, nationality, geography) [17]. Baseline information is most efficiently presented in a table [39] which was the case in all studies in this SR. All studies measured the same patient baseline characteristics (sex, age, tumour type and size, Eastern Cooperative Oncology Group (ECOG) performance status, and number of prior treatments) thus ensuring patient characteristics were as similar as they could be at baseline.
All trials assessed patients over 18 years of age with an overall average of 44 years. The vast majority of patients were female although gender was not an inclusion/exclusion criteria in any study. Fong et al. [22], Tutt et al. [29], Kaufmann et al. [33] and Robson et al. [30] studies are multi-national while Balmana et al. [31] and Gelmon et al. [32] are multi-center studies so patients were assessed from all over the world (EU, Australia, Israel, Canada and USA). It is beyond the scope of this review to assess the benefits or downfalls of a multi-center or multi-national collaboration on clinical trials, but there is the question of confounding factors such as differences in equipment, methods, and personal opinions of different researchers between such diverse locations, compared to a study conducted in one central laboratory. However, multi-center studies are considered excellent sources of evidence for evaluating healthcare interventions [17, 42].
3.1.4. Sample Size
Large-scale, multi-site RCTs are often required to establish the superiority of one treatment over another [17]. However, very few of the trials in this SR had large sample sizes, which is understandable because the condition under investigation (BC patients with BRCAm) is not usual to the general population [38]. All studies except Tutt et al. [29], Kaufman et al. [33] and Robson et al. [30] have a very small sample size of patients (3-17 individuals), leading to the potential that some results from small cohorts could be due to chance. Fong et al. (2009) had only nine BC patients and of those, only three had BRCAm, so these are very small numbers indeed. Small sample sizes are a potential limitation of the papers included in this SR because large samples are necessary to detect small differences in effect size [39]. Future large-scale trials are required to provide more externally valid results, because trials with inadequate samples sizes (like most in this SR) are associated with bias, as having too few patients runs the risk of missing statistically significant findings [21] due to type II errors.
3.1.5. Blinding and Randomisation
Being aware of which interventions the control and experimental groups are receiving may introduce bias on the part of the subjects and researchers [17]. Blinding the groups of individuals (participants, researchers, or data collectors) who can introduce bias into a trial is crucial for successful randomisation [39].
Robson et al. [30] OlympiAD trial is the first and only trial in this SR to be randomised. However, this trial was also open label (unblinded) which may influence participants' compliance with the intervention, or risk participants dropping out of the trial. Participants may respond differently if they know which treatment group they are assigned to (e.g. by responding more positively when know they are receiving the new treatment) [17].
Unfortunately, all studies in this SR are open-label, so participants could introduce biases as above, and researchers may also introduce different biases. Moher et al. [39] suggest that unblinded researchers may assess subjective outcomes differently. Unblinded data analysts might introduce prejudice through a biased selection of positive results, or by making decisions to remove patients with unfavourable results from the analyses. Random assignment is the preferred method to assign interventions to trial participants, and when properly implemented, it eliminates bias in the assignment of treatments [39]. Without randomisation, treatment comparisons and results can be biased, even subconsciously [17]. This lack of randomisation and blinding in all the trials in this SR imposes a limitation of this SR because randomisation is such as this is a crucial component of high-quality RCTs [39].
3.1.6. Dropout Rate and Patient Losses
If patients do not complete the study then data is lost and outcomes not fully assessed [21]. This may be because of AEs, voluntary withdrawal, secondary disease or death. The rate of retention of patients is very important in any study. If a large percentage of participants withdraw from the study or choose to drop out for any reason, the results are likely to be different than if all of the participants had remained in the study, since patients with specific characteristics may be more likely to drop out or be forced to discontinue [35]. ‘Intention to treat’ analysis was used in all studies, which means patients were analysed in the group to which they were originally assigned.
It is not uncommon for participants not to complete a study-they may drop out for their own reasons or be withdrawn from active treatment due to adverse effects (related to the treatment or otherwise) - and therefore their outcomes are not assessed at the end of a trial [39]. Despite reporting of several patients dropping out in the trials in this SR, all patients were accounted for, and their results were included in the analysis if they had had at least one dose of olaparib, given that ORR had to be confirmed at a second assessment four weeks later.
3.1.7. Publication Bias
Every researcher has an ethical obligation to publish complete and accurate research results of all trials with human participants [43, 44]. However, for many reasons, not all research results are published in an accurate way, and in some cases, they are not released at all [45]. Publication bias may occur after manuscripts are submitted to a journal, as well as before. Publication bias creates a false impression on the reliability of these clinical trials and also affects the clinical conclusions about the best treatments, which is an issue for evidence-based practice [45].
A clear finding from this SR, based on the results of all but one study [22] (as this study was published prior to AZ acquisition of the company that made olaparib) is the risk of publication bias. Risk of publication bias is particularly highlighted in Robson et al. [30] trial, as the 2.8 months, PFS benefit was measured by blinded independent central review. However, it is interesting to note that when the same data was assessed by the investigator, the PFS benefit of olaparib was 4 months (7.8 months PFS in olaparib versus 3.8 in a standard). The authors say their 4 months is similar to the blinded central review result of 2.8 months, but there is a 1.2-month gap which is certainly significant when the independent review result was only initially 2.8 months. Another potentially misleading statement by Robson et al [30] is that the risk of disease progression or death was 42% lower - however, the results show patients have not actually lived for longer when taking olaparib versus TPC so this suggests there may be some bias reporting.
Almost all of the research into olaparib is funded by one pharmaceutical company (AZ) or undertaken in collaboration with them, or assistance given with writing up [31-33], so there is a need to assess the level of bias in the reporting and publishing of these trials. In all of the trials in this SR, AZ was the major sponsor with researchers and authors having connections to AZ, or will benefit in other ways if olaparib proves to be a success. In Robson et al. [30], AZ was responsible for overseeing the collection, analysis, and interpretation of the data. The manuscript was written with medical-writing support funded by AZ, with critical review and input from authors [30]. Although in no way to question the integrity of AZ as a company, or of any of its employees or researchers, it may not be helpful in establishing the clinical efficacy of olaparib for one company to so completely dominate the research agenda. It is difficult to see how this may be ameliorated, however, given the huge expense involved in the development of new medicines. We note and commend the willingness of key personnel, mentioned above, to discuss their research with us in relation to this systematic review. Recent developments in clinical trials registration and in publishing, including transparent reporting of potential conflicts of interest, are also welcome in this regard.
3.1.8. Ethics and Consent
Beauchamp & Childress [46] suggest that there are fundamental ethical principles that all researchers should abide by including: autonomy, beneficence, non malevolence (do no harm), and informed consent, as well as the right for participants to refuse to participate or change their mind without prejudice, the right to revoke their permission to use their data, and even how their data is anonymised and accessed [17]. The World Health Organisation (WHO) advisesthat authors of SRs of primary research identify how these principles were put into practice in order to protect the participants [47]. However, of the seven trials of this SR, major ethical weaknesses were found in three. There is no patient consent mentioned we in three studies [22, 28, 33]. Informed consent by study participants is normally required in all intervention studies [39], and without full documentation of ethical considerations, concerns are raised because without proof participants understood the nature and stage of the disease being studied, the study may include persons vulnerable to harm from the study intervention, and this raise issues as to whether these studies satisfy WHO as above, as well as other legal and ethical norms [39].
3.2. Methods of Data Collection and Analysis
Critical appraisal of the quality of RCTs is possible only if the design and methods of data collection and analysis are thoroughly and accurately described in the published literature [39]
Despite the heterogeneity of the studies in this review, all methods used in all trials were described thoroughly. Recognised standard methods of data collection and analysis were used, and in many cases the same methods were used in other trials, enabling valid and reliable comparisons to be drawn between the studies where it was possible [17].
Both dose-finding trials [22, 31] used a standard 3+3 dose finding method - this means treating at least three assessable patients per dose for one cycle, with a doubling of the dose in the absence of AE of grade 2 [22] at that dosage or grade 3 [31]. Each cohort was expanded to six or more when olaparib dose was increased or if one dose-limiting toxicity (DLT) was observed at a given dose. A dose was considered the MTD if two manifestations of DLT were observed at that dose during the first treatment cycle. A drug related AE of grade 3 or 4 occurring in the first cycle was considered a DLT.
Another finding of these studies was that they all measured Objective Response Rates (ORR) (CR or PR) using the recognised Response Evaluation Criteria in Solid Tumours (RECIST] [49], and all studies assessed radiological response by means of Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) which were carried out at least 28 days initially [22, 28, 29, 33]. An additional strength of Robson et al. [30] was that the authors also assessed overall survival (OS) although this was not the primary endpoint (PFS was primary endpoint), and assessed the time to second progression event or death after first progression event.
In every study, the analyses of measurable response weredone for all patients who had had at least one dose of olaparib. Importantly for the precision of results, all trials ensured 95% confidence intervals [34], which were measured using the recognised Wilson score method as recommended by Newcombe and Altman [50]. Confidence intervals (CIs) are the best indicators of the precision of the result values [35], as a 95% CI is a range of values within which the reader can be 95% confident the true value lies for the study population.
The olaparib dose used in Robson et al. [30] study is different from the FDA approved 400-mg twice daily dose in capsule formulation used in all the previous trials in this review. Tablets give a higher exposure while the adverse effects are not expected to be different [30], however how much difference the dosage and delivery method of olaparib makes is outside the scope of this review but is something that should be determined by future investigations and analysis.
Where BRCAm analysis was done, the validated and approved external central reference laboratory Myriad Genetic Laboratories carried out the analysis (this is the laboratory that was granted the sole rights to perform these tests since 2014). A weakness of Balmana et al. (2014) trial is that they did not originally test for BRCAm. However due to a protocol amendment, BRCAm status was collected for patients who had previously been tested for gBRCAm but as these patients BRCAm status is not centrally validated by Myriad Genetic Laboratories results must be treated with some caution. The same caution applies to Kaufman et al. [33] results as patients’ BRCA status was not centrally validated.
Data was collected and analysed wholly or in part by a third party in some of the trials [22, 29, 33] which leads to a reduction of potential bias in these studies. In others, however, data collection and analysis was done by AZ themselves, with interpretation by the authors in collaboration with the sponsor. Gelmon et al. [32] admitted that there was no independent review of responses done in her trial, but where statistical analyses weredone [29, 31] a recognised system using SAS software (version 8 or 9) was used.
PFS (assessed by RESIST) plots were created using the recognised Kaplan Meier method, and Robson et al [30] also used Kaplan Meier method to generate time to event curves. Additional validity and reliability for adverse events (AE) data wereprovided by an independent data monitoring committee who reviewed Tutt et al.’s [29] safety data, but this was not something mentioned for AE in any other trial. However, all AEs in all trials were graded according to the standard and recognised Common Terminology Criteria for Adverse Events (CTCAE), [51].
Overall, the approved methods used for data collection and analyses in these studies abide by the CONSORT checklist, with results for all analyses performed, including subgroup analyses [39].
3.3. Study End Points
In oncology trials, an increase in overall survival (OS) is the most convincing measure of drug efficacy and patient benefit [52]. Measuring OS requires extended follow-up with large numbers of participants, so OS results may be confounded by the use of rescue therapies during this time. To address this limitation, recent studies have introduced a range of intermediate endpoints such as Progression-Free Survival (PFS) and time to Progressive Disease (PD). Researchers in the RCTs in this SR subcategorise ORR in terms of Complete Response (CR), Partial Response (PR) or Stable Disease (SD) [52].
Overall Survival (OS) is the gold standard for oncology trials, followed by PFS and then ORR [39]. However, all studies in this SR assess PFS as a primary endpoint rather than OS. PFS seems acceptable when survival is good as it takes a long time to get the OS data, but for patients with BRCAm BC who have already progressed on two or more previous lines of therapy (which all these patients in these trials have), then the OS is short. It would have been far more reliable and more valid to have been able to assess the OS of BRCAm patients taking olaparib for BC. Although Robson et al. [3] did assess OS in their study, the primary endpoint was PFS so despite the OlympiAD trial meeting its primary endpoint, without the full data on OS the true clinical effectiveness of olaparib cannot be certain.
One weakness of RCTs is that they tell us nothing on their own about the patient’s experience [21]. There are also concerns regarding whether olaparib improves meaningful outcomes for these patients other than clinical effectiveness, such as appropriateness and feasibility [42]. Evidence-based medicine requires any external evidence to be integrated with an individual patient’s clinical state and personal preferences [40] which was not the case for the trials in this SR (except 30, where Quality of Life was included as a secondary study endpoint). Therefore, future trials should go on for longer and include OS results, which would mean data for assessing the effectiveness of olaparib is more reliable and free of bias. However, as explained by AZ’s medical officer, trials could go on for a very long time, e.g. Olympia. Given the apparent benefit of olaparib for these patients, it could be argued that it is better to have more results sooner, rather than wait for confirmed OS data which may take years, as questions about some therapies cannot wait for large-scale, long term trials to be conducted [40].
3.4. Overall Synthesis
The major limitation of the studies included in this SR is their lack of randomisation and lack of proper control/placebo arms. Even in Robson et al. [30] randomised phase 3 trial which did have comparator arms, neither the patients nor the researchers were blinded. Another major limitation of this SR is the small number of trials to date, and within those trials, the small sample sizes who fit the criteria of having BC and BRCAm - there are cohorts of 17 or less in over half of the trials [22, 28, 31, 32].
Despite patient demographics being consistent, and methods of data collection and analysis recognised, producing valid ORRs, the number of studies, the small sample sizes and heterogeneous nature of the trials in this SR prevented a statistical meta analysis being undertaken.
AZ did confirm that all studies they are aware of have been sourced for this SR, and despite a limited number of studies, it is clear from the studies included here that the evidence contains similar themes and overall there is a benefit of olaparib therapy (monotherapy or combination therapy) over standard therapy. Larger, longer term trials with more comparator arms should be undertaken if this hypothesis is to be substantiated.
The main ORRs are shown for comparison in Table 3 below.
The ORR was not measured in Van der Noll et al.’s [28] long-term safety study, so no results are shown, however, results of Lee et al. [6] combination study (did not meet all inclusion criteria for SR) are included for interest and comparison with Balmana et al. [31] combination trial.
Clinical effectiveness has been measured in all the trials in this review as the Objective Response Rate (ORR). Despite ORRs varying wildly from zero response [32], to 71% [31], the overall clinical benefit of olaparib appears to be greater and longer lived in BRCAm carriers compared to BRCAwt, and also when compared to standard chemotherapy treatments.
| Authors | Therapy | Phase | Type of Cancer | Olaparib Dose | No. of Patients(No. with BRCAm BC) | MTD (dose finding trails only) | Objective ResponseResults in BRCAm cohorts |
|---|---|---|---|---|---|---|---|
| Fong, 2009 | Olaparib | 1 | Solid tumours | 10-600 BID | 60 (3) | 400 mg BID | 33% |
| Tutt, 2010 | Olaparib | 2 | BRCAm breast cancer | 400 or 100 BID | 54 (54) | — | 41% at 400 mg BID, 22% at 100 mg BID |
| Gelmon, 2011 | Olaparib | 2 | BRCAm breast cancer and ovarian cancer | 400 BID | 90 (10) | — | 0% |
| Balmana, 2014 | Olaparib plus Cisplatin | 1 | Breast, ovarian, pancreatic, peritoneal cancers | 50-200 BID (continuous and intermittent dosing schedules) | 53 (17) | cisplatin 60 mg/m2 with intermittent olaparib 50 mg BID deemed tolerable but MTD not reached | 71% |
| Lee, 2014 | Olaparib plus Carboplatin | 1/1b | BRCAm breast cancer and ovarian cancer | 100-400 BID (continuous and intermittent dosing schedules) | 45 (8) | carboplatin AUC 5 with intermittent olaparib 400 mg BID was highest tested dose but MTD not reached | 87.5% |
| Kaufman, 2015 | Olaparib | 2 | Various BRCAm cancers | 400 BID | 317 (62) | — | 12.9% |
| Robson, 2017 | Olaparib | 3 | BRCAm breast cancer | 300 BID | 302 (302) | — | 59.9% |
Tutt et al. [29] reported 400mg BiD shows a near doubling of ORR when compared to patients on lower 100mg BiD (41% versus 22%), and median reduction in tumour size was of 30% compared to only 7% for the 100mg cohort suggesting the MTD is the most clinically effective. The presence of a dose-response result is recognised as an important criterionfor believing there to be a reputed cause and effect relationship.
Long-term monotherapy also reported good results, with Van der Noll et al. [28] reporting 43% of all patients still on the study at the time of data cut off, including four patients who had been on the study for over 2 years - 43% with CR, 22% PR, and 29% SD. Only 5% of patients showed PD.
When compared to standard chemotherapy, olaparib showed improved clinical effectiveness. In Robson et al. [30] trial comparing olaparib with standard chemotherapies, ORR in the olaparib cohort doubled (59·9% compared with 28·8%), and CR was seen in 9% of olaparib group versus just 1.5% in TPC group. Further encouraging results show that the tumour reduction of -45.1% for olaparib arm compared to 14.8% for TPC arm.
The studies that reported zero or low ORR are important to consider. Gelmon et al. [32] saw no ORR in BC cohorts at all, while Kaufman et al. [32] saw ORR of only 12.9%. Despite no ORR to olaparib monotherapy for any of the BC patients, it is important to note that there was a clinical benefit. SD was seen in 63% in BRCAm cohort compared to 13% in the BRCAwt cohort. PD in BRCAm cohort was less than half that seen in the BRCAwt cohort (38% versus 80%), and despite not being included in the final confirmed results, 50% of BRCAm patients saw tumours reduce in size by more than 30%, which definitely warrants further exploration. Clinical benefit was also derived in one trial [33] as 47% of the BRCAm BC cohort maintained SD which was higher than any other cancer type in this trial.
Overall, whilst the number of trials to date is small, and the patient cohort size within those trials is often even smaller, the overall results suggest that olaparib does show clinical effectiveness for BC patients with BRCAm.
In Fong et al. [22] study, the clinical benefit rates for patients with BC and BRCAm were 69% in platinum-sensitive patients, 45% in platinum-resistant patients, and 23% in platinum-refractory patients, suggesting a link between platinum resistance and olaparib response; that platinum-sensitive patients will benefit the most from olaparib therapy. In Gelmon et al. [32] study, trial responses were also consistent with prior platinum sensitivity as post hoc analysis showed activity mostly in patients with platinum-sensitive disease. This hypothesis gains strength as the studies continue with 62 platinum resistant BC patients [33] with at least three lines of prior therapies (median of 4.6) resulting in ORR of 12.9%, compared to minimum of 1 treatment and median of three treatments in another [29], which resulted in ORR of 41%. In addition, although the ECOG status was the same in both trials (ECOG range 0-2), Kaufman et al. [33] had more patients with ECOG of 2 (which means they have longer cancer history which tallies with the higher pre-treatments) than Tutt et al. [29]. Gelmon et al. [32] results showed zero response to olaparib but 70% of the BRCAm BC patients had had at least 3 prior lines of chemotherapy, so were also heavily pre-treated, while in Robson et al (30) trial, the superiority of olaparib was even more pronounced in patients without prior platinum exposure. Of particular note in Kaufman et al. [33] trial, there was a doubling of response in BC patients (20% versus 9.5%) who had not had prior platinum treatment.
Consistent across all the trials in this review, the clinical benefit rates seem to correlate to platinum sensitivity, suggesting that platinum sensitivity may be a surrogate marker for sensitivity to olaparib treatment. Indeed looking at the results of all studies it appears that platinum sensitivity may be enough to predict response to olaparib. For all the trials that measured it, there was also a consistent association between clinical benefit and platinum-free interval (the period between last platinum therapy, and disease progression) which should be explored in longer term studies in the future.
Mechanisms of resistance are not measured in any of the trials and areoutside of the scope of this SR, but it is certainly something for future investigation in order to understand the relationship between prior platinum exposure and response to olaparib. Unfortunately, in Robinson et al. [30] trial, there was no platinum-based chemotherapy to compare with olaparib. It is encouraging that efficacy was seen in patients with prior platinum exposure, however, it was outside the scope of the trial toassess the effectiveness of olaparib in patients with the platinum-resistant disease. Considering the high success rate seen by Lee et al. [6], carboplatin as a control arm in Robson et al. [30] trial would have been the ideal control to test the efficacy of olaparib compared to carboplatin. Although previous treatment with carboplatin was allowed in Robson et al. [30] trial, only 14% of patients had received platinum previously and subgroup analysis shows that the superiority of olaparib was even more pronounced in patients without prior platinum exposure. Further research into the link between platinum sensitivity and response to olaparib is warranted.
A consistent theme throughout all the trials in this review is that despite the majority of patients being affected by AE’s grade 1-2,and 40-50% of all patients being affected by grade 3 AEs for all of the olaparib monotherapy trials in this review, the drug was generally well tolerated causing mainly mild toxicities such as nausea, vomiting, fatigue, headache, cough, and a low incidence of myelosuppression. No secondary malignancies were seen as a consequence of taking the drug in any trial. The toxicities did not differ based on germline BRCAm status of those patients except for in a long-term safety study [28] where BRCA2m patients had 100% CR (but this is only three patients so could be due to chance).
In combination, olaparib seems to improve the therapeutic responses of patients with different cancers to platinum [6, 31], although toxicities of these drugs were also increased. Olaparib seems to enhance the anti tumor activity of platinum chemotherapies. The combination of olaparib with platinum appears a promising approach to increase efficacy and warrants further investigation in the future.
4. DISCUSSION
This is the first systematic review looking at the effectiveness of olaparib in BC patients with BRCAm, and there are several themes arising from the research into these trials, as well as several limitations. All the studies in this SR used quantitative research designs, and all were examining olaparib’s effectiveness in patients with BRCAm and associated cancers, measured by objective response rates and adverse effects. This SR focusses on the results for patients with BC.
4.1. Interpretation of Results and Limitations of this Review
Despite the author abiding by a strict standard process in order to write this SR as objectively as possible, there will always be a difference in interpretation between Kmet et al [23], CASP and hierarchy of evidence. Of particular note for this SR, is that the Kmet et al. [23] method may be flawed as all except one of the trials were non-randomised - so for most papers, there were irrelevant questions in the Kmet et al. [23] score sheet.
5. If interventional and random allocation was possible, was it described? NA
6. If interventional and blinding of investigators was possible, was it reported? NA
7. If interventional and blinding of subjects werepossible, was it reported? NA
So quality guidelines such as Kmet et al. [23] can be inconsistent in how they rate quality of evidence because scoring in this instance means that what are effectively poorer study designs of the RCTs in this review (non-randomised and open-label, small sample sizes) can score as highly as a large sample size fully blinded RCTs using Kmet et al. [23] method.
Trial designs in the studies included here are very heterogeneous with all except one being phase 1 or 2, meaning current research in this area is still in its infancy and, although two other phase 3 trials are currently ongoing, no results are yet available. Treatment and dosages are not always the same in all studies, and even within the same study dosages can be suspended, reduced, increased, or given intermittently. In dose-finding trials, there were reports of fluctuation in dosage and duration of therapies, with patients switching between dosages.
Despite the heterogeneity of the study designs, the studies did use recognised and established methods for conducting the intervention, data collection and analysis, providing valid and reliable results, so although the numbers of patients in the studies are small, and there are only a few studies to date, these results can be compared and an overview of the body of evidence can be assessed [40].
Robson et al. [30] OlympiAD Trial is the first and only trial in this SR to be randomised. This trial was designed to compare the efficacy and safety of olaparib with the efficacy and safety of standard Therapy of the Physicians’ Choice (TPC) among BC BRCAm patients. Randomisation was stratified according to previous use of chemotherapy, hormone receptor status, and previous use of platinum-based therapy. 302 patients were randomised 2:1 to olaparib (205 patients) or chemotherapy of the physician’s choice (97 patients), consisting of either capecitabine, vinorelbine or eribulin, although the ideal comparator would have been carboplatin. Future trials should have multiple arms, including platinum arms and placebo control. It is of interest to note that the ongoing OlympiA study has randomised patients with BRCAm to olaparib or placebo for 12 months, after completing surgery and chemotherapy (NCT02032823), but these patients are surviving for a long time so the endpoint for this study has not yet been reached, so we wait to see these results when they are available.
It is important that the demographics of patients at baseline is similar [53]. Patient demographics in all arms were similar in all trials, except for the unavoidable differences in individuals’ prior treatment regimes. Some patients were more than three times more heavily treated than others. All the studies except Robson et al. [30] and Gelmon et al. [32] had patients with a median of 3 prior chemotherapies. Robson et al.’s (2017) patients had a maximum of two prior treatments and Gelmon et al. [32] patients were much more heavily pre-treated with a median of 4.6 chemotherapies.
In particular, when reading Van der Noll et al.’s [28] data for this long-term safety study, it is important that baseline characteristics are taken into account as most patients had already shown anti-tumour response during previous combination study (19 out of 21 had to benefit, one had PD and one had a non-evaluable response). There is also potential selection bias over time, as only patients that tolerated the treatment actually remained on the study, however, none of the patients that went off study did so because of AEs. The Berger-Exner test detects selection bias but has not been widely utilized in practice [54]. One reason for the non use of this test may be a lack of information regarding its accuracy but Michenautsch et al. [54] concluded that the Berger-Exner test is generally accurate for identifying selection bias so this could be utilised to avoid the same potential bias in future long-term safety studies.
RCTs where baseline characteristics of patients in each are balanced (which all of these trials did achieve) can ensure high levels of internal validity [53]. However, despite establishing that everything that could be done to ensure baseline characteristics are as similar as they could be, it must be remembered that none of the studies in this SR used a randomised double-blinded method in their experimental design, so the risk of bias must always be considered, and this compromises their external validity.
Small sample sizes are also a limitation of the papers in this SR and all results from studies with small sample sizes should be considered with caution. One of the three patients with BRCAm BC in Fong et al. [22] trial had a CR lasting more than 60 weeks on olaparib 200 mg twice daily, one had SD, but one left the trial. Of the 26 patients in Gelmon et al. [32] BC cohort, 11 had BRCAm (although this changed at interim analysis to 10 as a result of baseline BRCAm testing which found one BRCAm BC patient actually did not have BRCAm, and so switched cohort). The poor sensitivity of BC patients in Gelmon et al. [32] trial could simply be due to chance because of the very small sample size (10 patients) in this study. Consistent with many of the trials in this review, the cohort numbers in Van der Noll et al.s [28] safety study are very small which can lead to bias [21, 39, 40].
A much larger study, so potentially one with greater power [17, 38] by Kaufman et al. [33], published results of a single arm, non-randomised phase 2 trial with 298 patients with recurrent BRCAm cancers, to discover the efficacy and safety of olaparib as a monotherapy in platinum-resistant OC, heavily pre-treated BC, pancreatic cancer and prostate cancer. For all patients, ORR was 26.2%, but only 12.9% for BC patients, which is low when compared to Tutt et al. [29] 41% for same BRCAm breast cancer patients. This inconsistency could be explained by the more heavily pre-treated nature of Kaufman et al. [33] patients, but it could also be a more precise result due to the larger sample size.
It is important to consider timescale and dosing [17] as well as patient drop out [21] in studies using experimental designs. All trials gave treatment cycles of three or four weeks initially and ran for at least six cycles (24 weeks). Not all patients finished six cycles (only 12% in Gelmon et al. [32] heavily pre-treated population), but in some cases, patients were still on trial at data cut off [32, 33] with some surviving over three years [28, 31]. The median duration of treatment in BC patients ranged from 56 days [32] heavily pre-treated population) to [33] days for combination with cisplatin [31]. Additionally, it seems that the higher dose of olaparib is tolerable over a long time, as Tutt et al. [29] patients, treatment exposure ranged from 11 weeks in cohort 2 (taking 100mg BiD) to 163 weeks in cohort 1 (taking 400mg BiD).
Although results are highly variable, it is interesting to note that those patients who survived the longest (over 3.5 years) were on maintenance therapy after receiving combination therapy. For those patients who continued on olaparib monotherapy after a combination therapy [28] the median treatment time of olaparib monotherapy was 52 weeks, with a range of 7 weeks to 183 weeks. It is important for future studies to include long-term follow up assessments in order to realise the sustainability of olaparib so that both the short-term and the long-term outcomes of olaparib can be determined [35].
In Gelmon et al. [32] trial three participants results were not confirmed as OR because of the absence of confirmation at their next visit (three BC patients (two BRCAm and one wt). Given the small numbers in the BC BRCAm cohort in this trial (10 patients), had the results of these three BC patients been confirmed and included in the results, the data would have looked very different, and consistent with findings of other studies in this review. This study design is certainly something reexamined with larger sample sizes in order to provide more reliable results.
Fong et al. [22] also had one BRCAm patient who dropped out with no given reason after one week so no ORR was recorded. Further details on this patient would have been useful as they may have experienced an acute exacerbation of their illness or AE to treatment. Due to the lack of information given [22], it was not possible to ascertain why this participant dropped out. This information is crucial because this patient may have been different to the other BRCAm patients in the study [39] but these were early days before BRCAm benefit in humans was only beginning to be noticed or acted upon [22]. There will always be patient drop out in RCTs, especially in females diagnosed with aggressive cancer affected by strong emotional involvement [55], therefore measures should be taken (such as counselling and better communication between researchers and participants) to minimise drop out rates in future, and account for missing data, in order for the findings to be valid and generalisable.
In Kaufman et al. study [33], BC and OC patients were moved between cohorts after centrally validated BRCAm testing was performed. Like Balmana et al. [31], Kaufman et al. [33] trial has a weakness in that no central validation of mutation status was done before enrolment. The authors of that study believe that the chance of miss-classification was low, however several patients were moved from one cohort to the other in Gelmon et al. [32] trial after BRCA status was subsequently confirmed by Myriad central laboratory, meaning that this may be an optimistic view, and therefore further trials are warranted where patients BRCAm status is centrally validated prior to a study commencing.
A point of interest resulting from one trial [31] of those patients who continued single agent olaparib monotherapy after the end of the combination trial - is that SD (>1 year) occurred in five breast cancer patients. Also, of all patients on the trial with various cancers, two had responses lasting for over three years, but Balmana et al. fail to disclose whether these were BC or OC patients.
Interestingly, Van der Noll et al. [28] long term safety study showed reduction of severity and frequency of AEs over time. The most common AE was bone marrow suppression, highest at baseline, suggesting a possible carry over from the combination study with chemotherapy. The only haematological AE that persisted was anaemia, warranting further investigation of olaparib’s role in anaemia, but encouragingly for olaparib therapy, no patients had to omit or discontinue due to AEs. One specific weakness of Van der Noll et al. [28] study is that there appears to be a discrepancy in the text versus the table. The authors say in the text that in total 8 out of 16 (50%) patients with known BRCA mutations had to come off study due to PD. Whereas in Table 4 (in the original text), it shows only one patient (8%) having PD. One patient would be more consistent with previous and subsequent data for olaparib monotherapy. There is also a subsequent error in the text, referring to the same 8 patients there were eight patients with BRCA mutation who did show disease progression. The authors have declined to comment despite contacting them several times
| • High quality— Further research is very unlikely to change our confidence in the estimate of effect • Moderate quality— Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate • Low quality— Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate • Very low quality— Any estimate of effect is very uncertain |
Robson et al. [30] trial had comparator arms, which showed more serious AEs occurring more often in the TPC cohort. Grade ≥3 adverse events rate was 36.6% in the olaparib arm and 50.5% in the TPC arm. In addition, 4.9% in the olaparib arm discontinued the study because of AEs versus 7.7% in the TPC arm. The median treatment duration was more than double in the olaparib arm (8.2 months) than in the TPC arm (3.4 months). Interestingly, the most common grade 3 or worse AE’s were caused by anaemia (16·1% in the olaparib group vs 4·4% in the TPC group) again raising questions about olaparib’s role in anaemia (which is beyond the scope of this SR but worthy of future investigation).
Despite the apparent success of olaparib monotherapy, combination studies with cisplatin found hematologic toxicity too great, and the tolerable schemas required decreasing the standard doses of platinum and were limited by Dose-Limiting Toxicities (DLTs) [31]. This increase in myelosuppression could be attributed to an increase in the sensitivity of rapidly dividing cells to the toxic effects of platinum by olaparib. A schema of intermittent olaparib (50mg BiD, days 1-5) with cisplatin 60mg/m2 was deemed tolerable for further development, and this should be explored in the future. Although not a trial appraised in this review due to its focus on biomarker analysis, it is noteworthy here to compare the results of Lee et al. [6]carboplatin/olaparib combination therapy trial, which reported 87.5% ORR (Table 3). This is the most impressive ORR to olaparib in BRCAm cancers found during the research for this SR, and further investigation with different combinations of olaparib/ platinum is needed in order to achieve anti-tumor efficacy with well tolerable regimens, which could greatly improve prognosis and disease outcomes. Interestingly, Lee et al. study [6] with carboplatin/olaparib combination therapy reported less frequent AEs, but this could be because a tolerable dose was found more quickly.
All studies except [22, 28, 33] state that patients had provided written informed consent. Although there is no mention of ethical considerations in one study [28], this is a long-term safety study following on from a phase 1 trial, so approval may have been sought previously for the phase 1 stage. All studies had been approved by review boards and ethics committees for each trial centre, and done in accordance with the recognised Good Clinical Practice guidelines and the declaration of Helsinki, which shows that each study has considered and addressed the wider ethical concerns. An additional strength of three studies [29-31], is that they are the only papers to state that they abide by the sponsor's policy on bioethics [48]. However, without written consent from each patient, there remainsconcerns about whether participant’s autonomy, confidentiality, anonymity and personal safety have been considered, and we cannot know if the authors have ensured their patients understood what the trial was, or agreed to take part [17, 21, 38, 39].
Another ethical consideration, given the apparent success of olaparib as a monotherapy, is a potential limitation for future RCTs. Giving olaparib to one cohort whilst giving standard (believed to be inferior) alternative therapy or placebo to another cohort can be thought unethical [56]. For example, a non-randomised study suggested that multivitamin supplementation (which included folic acid) during pregnancy could prevent neural tube defects in developing embryos [57]. Despite the study being deemed flawed [56], ethics committees believed it was unethical to deprive patients of this potentially beneficial treatment. It appears unlikely that this currently applies to olaparib, due to the heterogeneous nature of the results so far, but it gives another reason to look forward to the results of the randomised double blinded phase 3 RCT results due to be released later this year (OlympiA and neo-Olympia).
All RCTs in this SR reported a combination of multiple single endpoints (CR, PR, PFS, PD) and all endpoints, primary or secondary, should be identified and completely defined [39], which they were. Robson et al. [30] trial primary endpoint was PFS, but the authors did also report OS where possible. At data cut off (December 9, 2016) 36 patients were still receiving olaparib and three were still receiving standard therapy. It would be interesting to know whether these patients are still alive and if not, what the OS was. However, OS results could be unreliable because, after first disease progression, patients in TPC group received treatment with PARPi, platinum and other chemotherapy while still receiving the assigned treatment for this trial (supplementary appendix of Robson et al. 2017 (30]). Additionally, Robson et al.’s [30] trial is the first trial to measure time to second progression and further analysis should be done in future for PFS2, and even PFS3, as it could be hypothesised that olaparib as a maintenance therapy extends the period of time in between chemotherapy treatments, which is better for patients from a toxicity point of view.
Endpoints are equally important to patients but no trials documented patient preference, or whether patients weigh the importance of each endpoint differently [58]. Measuring QoL is important because it improves understanding of a patient's problems by enabling communication between physician and patient [59]. Robson et al. [30] OlympiAD trial did measure quality of life (QoL) alongside clinical effectiveness. Despite being outside the scope of this review, it is interesting to note that the QoL data showed a meaningful improvement in health-related quality of life measures in the olaparib arm. Briefly, the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire QLQ-C30 has 100 points, developed to assess the quality of life of cancer patients [60]. In the OlympiAD trial [30] a drop of 10 points or more was considered a clinically meaningful decrease, which was not reached in the olaparib group and took 15.3 months in a standard therapy group. The onset of response was similar for both groups, which is important when deciding which therapy to give patients in future, especially given the higher ORs and lesion shrinkage rates with olaparib versus standard therapy [30].
At the time of writing, the complete OS data is not available due to the authors report that it is not yet mature, but a pre-planned interim analysis revealed no difference between olaparib and TPC cohorts (19.3 vs 19.6 months, respectively), likely because of the high degree of cross-over during treatments for PD as mentioned previously. However, if olaparib does extend PFS, but does not extend OS, then it will become important in future trials to assess patients’ QoL during this time, in order to assess additional benefit of olaparib to patient’s lives, rather than just clinical effectiveness.
4.2. Implications Regarding Hierarchy of Evidence and Clinical Effectiveness
Most quantitative investigators argue that RCTs should be central to evidence-based decision making, since, in terms of clinical effectiveness, it is necessary to know the cause and effect relationship between and intervention and the results, based on valid and reliable outcomes [61, 62]. RCTs are a way of assessing the effectiveness of different treatments to find out which one works best relative to others [39] and are considered to be the ‘gold standard’ in this regard [63].
However, as we have seen, the trials assessed here have several limitations: small sample sizes, single arm, non-randomised and unblinded designs, imprecision and possible reporting bias. Additionally, there is variation in some methods and results between studies, resulting in a lack of statistical analysis, all of which weakens the strength of the evidence contained within this SR. Guyatt et al. [64], highlights that a high risk of bias can mean studies are rated down as a lesser robust quality evidence when compared to the gold standard RCT, which has been shown to be the case for the studies in this SR. From a post-positivist stance, Mantzoukas et al. [65] discourage an acceptance of RCT as the ‘gold standard’ in some circumstances because they cannot accommodate the diversity of patients and real situations faced in daily practice, and therefore the trials included in this SR illustrate pragmatism in design and conduct which has allowed scientists to begin evaluating the effectiveness of olaparib, but has not yet delivered a conclusive result.
However, CEBM says that not all SRs of heterogeneous RCTs need cause concern, and not all heterogeneity of results is statistically significant. Overall, despite RCTs currently being at the heart of evidence-based practice [66], the conclusion of this SR suggests that RCTs being near the summit, and SRs of RCTs being the pinnacle of the ‘hierarchy of evidence’ remains a controversial issue. At the heart of this conclusion is the basis that the development of the RCT is to discover an intervention’s ‘effectiveness’. The function of the trials in this SR is to determine whether olaparib is clinically effective: does it ‘work’? What are the ORRs of olaparib as an autonomous treatment? These studies included in this SR have a clear concept of whether olaparib ‘works’, as the clinical effectiveness is measured, compared and analysed. There are further questions about whether olaparib ‘works’ for patients, and whether it ‘works’ for the healthcare service which isnot answered in six out of seven (all except 30) of the trials in this review.
CONCLUSION
The aim of a SR is to find, analyse, appraise, and summarise the best available evidence related to a specific research question, in order to provide evidence-based answers for practice [67].
As this review is focussed on clinical effectiveness, results from studies used in this systematic review need the show clinical benefit (or not) to the patient and the response of a tumour/lesion to olaparib treatment. Results will ideally measure OS, PFS, and ORR, which include CR and PR and SD, and possibly the time until disease progression or other interventions (such as chemotherapy, surgery or death). Results also need to show AE resulting from the intervention.
Olaparib (made by Astra Zeneca), an oral PARP inhibitor, is tolerated as a single-agent in continuous doses up to 400 mg [22]. Olaparib has been shown to have promising activity in patients with metastatic BC and BRCAm and has been active as monotherapy in tumours with defective HR repair, specifically with BRCAm [29, 33]. Clinical activity with extended stable disease (SD) or tumour response (reduction) has been reported in BRCAm BC and OC [29, 32]. Phase 2 studies have confirmed the activity of olaparib monotherapy BRCAm patients with advanced BC [29] and those with OC [22]. Olaparib has been successful at inducing CR, PR and SD in BRCAm BC patients. Responses were often sustained, even in patients who were heavily pre-treated, making these results significant even in the absence of comparator arms in most studies.
In summary, olaparib as a targeted therapy for BC with BRCAm could potentially shift the paradigm of treating BRCAm BC away from chemotherapy towards targeting individual tumour biology. However, genetic complexity and ORR heterogeneity is currently a major limitation to the success of individually targeted therapy, and further research is needed.
Strength of Conclusion
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) is an approach used to assist a transparent approach to gradequality of evidence, and in line with GRADE criteria (Table 4), the quality of evidence in all of these studies would be graded as low quality because further research is very likely to have an important impact on our confidence in the estimate of effect (Table 4 below). Only one [30] had a comparatively large sample size (302 patients with BC and BRCAm) and used comparator arms with randomisation, so this trial could be graded as ‘moderate quality’ because further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate [68]; (Table 4 below). Overall, the seven studies together as a body of evidence would be considered ‘low quality’ according to the GRADE criteria shown in Table 4.
Limitations
There is also the matter of subjectivity to consider. Despite this being a quantitative SR with quantitative methodology and methods, there is still room for personal subjectivity from the point of view of the reader. Are the papers in this review the ones someone else would rank as best answering the question: ‘What is the current effectiveness of olaparib for breast cancer patients with BRCAm?’ Are the methods and methodology used in this review ones that the reader agrees with or not. However, if GRADE is used consistently, then the advantages of simplicity and clarity outweigh these limitations.
Quality of evidence is subjective and judgments about evidence and recommendations in healthcare are complex, however despite the limitations regarding the quality of the evidence, the authors have been clear about how conclusions were drawn about the quality of evidence, and how it was synthesised in this review, explaining Kmet et al. [23], GRADE measurement and scoring techniques, as well as using the CONSORT guidelines for reporting of clinical trials in order to assess the quality of the trials in this SR. Although poor quality studies may lead to biased outcomes [69], and the conclusion of this review indicates the evidence to date is low quality, the researchers of the primary evidence included in this SR were rigorous in their methods and they reported their work in enough detail for others to assess its quality [70]. Given the evidence to date, the outcome of the seven studies in this SR does suggest that the desirable effects of olaparib do outweigh the undesirable adverse effects.
Recommendations for Future Research
Questions remain about how best to use olaparib - either in combination with chemotherapy or as maintenance alone. Combination therapies with olaparib in this SR are extremely limited in number and the selection of the best therapeutic partner is as yet unknown. Such limitations increase the uncertainty of these combination therapies and more studies of combination therapy are needed. A randomised six arm study (including a placebo) could help to define whether an olaparib monotherapy, or olaparib/cisplatin combination therapy, or olaparib/carboplatin combination, improveclinical efficacy versus either platinum agent alone. Additionally, combination trials with other chemotherapy, radiation, immunotherapy and molecular targeted agents should also be explored.
It would also be very interesting to test whether a sequential treatment of olaparib monotherapy, after positive response with a platinum-based therapy, would improve the duration of response compared with olaparib/platinum combination therapy. Responses were observed with combination therapy, followed by durable responses to olaparib monotherapy in patients with BC. These findingssuggest that olaparib might be a promising maintenance treatment following monotherapy or combination chemotherapy, which is a strategy that should be tested in future studies.
Olaparib/chemotherapy combinations to date indicate platinum therapies might have overlapping mechanisms of actions to olaparib and might, therefore, be limited by the shared mechanism of resistance. This warrants further exploration, beyond our current knowledge that BRCAm tumours are sensitive to platinum for the same reason they are sensitive to olaparib. Not all BRCAm tumours in this SR responded to olaparib, and some non-BRCA tumours did respond to olaparib despite, in some cases, prior platinum treatment. This means there is a possibility that crossover of mechanism may not be as clear as we think, and is an area of research that needs further investigation.
Currently, BRCAm is the most reliable biomarker used to select patients for olaparib therapy, whether in combination or not, but as we have seen, not all BRCAm tumours responded to olaparib, and some non BRCA tumours did respond to olaparib, suggesting synthetic lethal interaction with olaparib may be exploited beyond BRCAm. New biomarkers to predict response to olaparib is an area requiring more research in order to identify all patients who might benefit from olaparib. It is clear that if olaparib is to expand into a BRCAwt population, a greater understanding of the mechanism of crossover of resistance is required and robust indicator tests outside of prior platinum response are needed.
While it is clear that olaparib has beneficial activity in BC, the timing of its use remains in question, whether as monotherapy, in combination therapy or as maintenance therapy. Given the success of olaparib in advanced metastatic BRCAm breast cancer, future trials are also needed to study the role of olaparib in early stage disease.
Quality of life (QoL) is something to consider for future trials. Olaparib did have lower toxicities and improved QoL compared to other therapies. Trials so far have not demonstrated increased OS, in spite of PFS benefit. However, the future gold standard for assessment of the efficacy of olaparib should be a clinically meaningful improvement in OS and quality of life (QOL), so future trials must include these in their measured outcomes.
The application of olaparib, (and other PARPi) is an ideal example of the concept of personalised cancer care: identifying molecular and or genetic differences and exploiting them to improve patient therapy and care. Studies in this SR looking at effectiveness show olaparib as an effective, tolerable therapy that could be expanded to other patients groups with further research. However, going forward, we have to further decide whether this is both feasible and appropriate with regard to patient care.
Firstly there is the matter of price. Olaparib is an expensive drug costing $12,155 per month [71]. To put this into perspective, consider Robson et al. [30] 2.8 month PFS benefit which amounts to $100,000 per patient. Despite the QoL results, we must consider whether the taxpayer is ready to spend this kind of money per person, for a relatively rare type of BC, when there are so many more people with other BCs, and other chronic diseases (diabetes and heart disease for example) that impact on our health as a society. This may not be such an acute consideration in insurance-based health care systems but will be central to decision making in the UK NHS. This will require evaluation by the National Institute for Health and Care Excellence (NICE).
Secondly, genetic testing comes with its own set of problems. Despite genetic testing becoming more affordable, clinical support services would need more funding to cover other services such as genetic counselling for patients and their relatives, and communication with patients and their families about results and what it means for everyone who could be affected. Further research to identify patients and tumour groups that may derive therapeutic benefit from olaparib is required, and research into predictive biomarkers will be crucial to help with that stratification in the future. These are important factors to consider in evidence-based practice and making the decision of whether to implement olaparib therapy in a clinical setting [35].
Bryant & Benton [72] suggest that in our quest for the very best healthcare, we must constantly strive to use evidence-based practice and healthcare practitioners should use the research to challenge, improve, or evaluate practice [73].
Overall, the purpose of this SR is to answer the question ‘What is the current effectiveness of olaparib for patients with breast cancer and a BRCA mutation?’ The answer is that olaparib is an active PARPi that has shown significant positive clinical effects in patients with recurrent BC and BRCAm, and compared with single-agent chemotherapy, olaparib monotherapy improves clinical benefit and reduces AE in patients with BC and BRCAm.
However, much larger, longer term studies to investigate the different treatment effects of olaparib among sub-groups are needed, particularly for patients with prior use of platinum therapy, as would a direct comparison study to determine the relative efficacy of olaparib versus platinum therapies. Development of combination regimes is also needed to find the most effective schema with lowest AEs. More long-term safety studies and follow-ups after RCTs are required to establish long-term toxicities and risk of secondary malignancies. Future trials must include OS and QoL in their measured outcomes. In addition, there are several further areas where more research is needed in order to realise the full potential effectiveness of olaparib.
However, since 2009, olaparib has been investigated in BC with the most compelling results found in the subset of patients who harbour BRCAm. Based upon this fact, olaparib should be regarded as a clinically effective potential addition in the fight against BC for patients with BRCAm.
LIST OF ABBREVIATIONS
| AE | = Adverse Event/Effect |
| ASCO | = American Society of Clinical Oncology |
| AZ | = Astra Zeneca |
| BiD | = Twice a day |
| BRCA | = Breast cancer gene |
| BRCAm | = Mutant breast cancer gene |
| BRCAwt | = Wild-type breast cancer gene |
| BC | = Breast Cancer |
| CASP | = Critical Appraisal Skills Programme |
| CEBM | = The Oxford Centre for Evidence-based Medicine |
| CINAHL | = Cumulative Index to Nursing and Allied Health Literature |
| COCHRANE | = The Cochrane library |
| CR | = Complete Response |
| CRD | = Centre for Reviews and Dissemination |
| CTCAE | = Common Terminology Criteria for Adverse Events |
| DLT | = Dose limiting Toxicity |
| DNA | = Deoxyribonucleic Acid |
| ECOG | = Eastern Cooperative Oncology Group |
| EMA | = European Medicines Agency |
| EORTC | = European Organization for Research and Treatment of Cancer |
| FDA | = Food and Drug Administration (USA government dept.) |
| GRADE | = The Grading of Recommendations Assessment, Development and Evaluation |
| Grey material | = Unpublished material or evidence |
| JBI | = Joanna Briggs Institute |
| MTD | = Maximum Tolerated Dose |
| NHS | = National Health Service |
| NICE | = National Institute for Health and Care Excellence |
| OC | = Ovarian Cancer |
| OR(R) | = Objective Response (Rate) |
| OS | = Overall Survival |
| PR | = Partial Response |
| PD | = Progressive Disease |
| PFS | = Progression Free Survival |
| PARPi | = Poly (ADP-Ribose) Polymerase inhibitors |
| PICO | = Population, Intervention, Comparative intervention, Outcome |
| PROSPERO | = An international database of prospective SRs |
| PRIMO | = University of Plymouth Online Library |
| PRISMA | = Preferred Reporting items for Systematic Reviews and Meta-Analyses |
| PRISMA-P | = Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols |
| QoL | = Quality of Life |
| RCT | = Randomised Clinical Trial |
| RECIST | = Response Evaluation Criteria in Solid Tumours |
| SD | = Stable Disease |
| SR | = Systematic Review |
| TNBC | = Triple Negative Breast Cancer |
| TPC | = Therapy of Physician’s Choice |
| WHO | = World Health Organisation |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
This study is adapted from an unfunded MSc dissertation.
ACKNOWLEDGEMENTS
Declared none.


