All published articles of this journal are available on ScienceDirect.
Effects of Gargling with an Aroma Solution on Xerostomia, Halitosis, and Salivary pH in Hemodialysis Patients – A Randomized Controlled Trial
Abstract
Background:
Despite developments in renal replacement therapy, therapeutic fluid restriction reportedly induces xerostomia in 28.2~85.5% of hemodialysis patients, which causes serious inconveniences in their daily living and is detrimental to their quality of life.
Objective:
The purpose of this study was to identify the effects of gargling with an aroma solution (A-Solution) on xerostomia, halitosis, and salivary pH in hemodialysis patients.
Methods:
This study design was a randomized controlled trial. The participants of this study were 56 hemodialysis patients of E General Hospital in Seoul, Korea. They were divided into an experimental group (n=28) treated by gargling with 20 ml of A-Solution for 15 seconds and a control group (n=28) where pateints did not gargle with A-Solution, and data were collected from October 1 to November 15, 2013. The outcome variables were measured in the pretest and at 5, 30, 60, and 120 minutes in the two groups. The collected data were analyzed using SPSS (version 18.0 for Windows).
Results:
Xerostomia was lower in the experimental group than in the control group at each time point apart from the pretest and differed significantly in the interaction between groups and time points. Salivary pH and halitosis differed significantly between the experimental and control groups, across time points, and in the interaction between group and time point.
Conclusion:
The findings of this study suggest that aroma gargling is a useful oral-care intervention for solving oral problems experienced by hemodialysis patients such as xerostomia and halitosis.
1. INTRODUCTION
Chronic renal failure is a condition in which the glomerular filtration rate decreases and renal tissue no longer functions due to the progressive and irreversible destruction of nephrons. Patients with terminal renal failure can extend their lives only through renal replacement therapies such as dialysis and renal transplantation [1]. The number of patients receiving renal replacement therapy is increasing rapidly, and 67% of these patients receive hemodialysis [2]. Therapeutic fluid restriction reportedly induces xerostomia in 28.2~85.5% of hemodialysis patients, which causes serious discomforts such as difficulty in chewing, swallowing, and talking, in their daily living and is detrimental to their quality of life [3].
Xerostomia in hemodialysis patients is aggravated further by various factors, including chronic diseases, the side effects of specific drugs, old age, and the lower salivary flow [4]. Xerostomia with a reduced salivary flow rate and high saliva viscosity generally causes halitosis by increasing the volatile sulfur compounds produced by intraoral bacteria [5]. Other factors that induce halitosis in hemodialysis patients include periodontal diseases and the acidification of saliva. The salivary pH is closely related to salivary flow. When salivary secretion rate is low, salivary pH is decreased drastically, thus increasing halitosis [6]. Salivary pH is generally 6.5. Slightly acidic pH can suppress the growth and proliferation of Gram-negative and anaerobic bacteria, hindering the activation of enzymes necessary for putrefaction of amino acids whose end products have foul smell because these compounds contain reduced sulfur [7]. Saliva of individuals with xerostomia often has more acidic pH [8]. A lower salivary flow acidifies saliva by decreasing the level of bicarbonate in the saliva, and this acidification worsens halitosis by causing dental caries and increasing the amount of tongue coating and the activity of intraoral acidic bacteria [9]. Lower salivary pH and lower amount of saliva are associated with more halitosis [10]. Owing to the disagreeable bad or unpleasant odor emanating from the mouth air and breath, talking face to face and forming social relations with others are difficult [4].
Previous studies of the oral problems experienced by hemodialysis patients have been limited to surveys of the severity of halitosis [11], xerostomia [12, 13], and periodontitis [14], and interventions for oral problems such as gum chewing [15], acupressure [16], medication [17], artificial saliva [3], and psychological intervention [18]. Further research is needed to identify the most appropriate type of oral care for hemodialysis patients. Gargling with various solutions has been used as an oral intervention for cancer patients [19], surgical patients [20], healthy people [21], and the elderly [22], and mouthwashes that are commonly used in clinical applications include physiological saline, Betadine, Tantum, and Nystatin. These solutions contribute to oral care through exerting various effects on oral mucosa, such as cleaning, disinfection, sterilization, infection prevention, and antifungal effect. However, Betadine and Tantum solutions irritate the oral mucosa, Chlorhexidine turns the tongue and gum yellowish brown and is likely to change the sense of taste, and physiological saline, which does not have a sterilizing effect, has to be used together with other mouthwashes [23].
In contrast, aromatherapy, which has been studied recently from various perspectives, is a safe and effective method with few side effects, and the effects of aroma on the oral cavity include reduction of halitosis, mitigation of intraoral inflammation, sedation, analgesia, and anti-inflammation [24].
The purpose of this study was to apply aroma gargling to hemodialysis patients in order to examine its effects on their xerostomia, halitosis, and salivary pH, and to assess the effect duration of aroma gargling, with the ultimate aim of developing an oral-care intervention for hemodialysis patients. This study examined the effects of gargling with an aroma solution (A-Solution) on xerostomia, salivary pH, and halitosis in hemodialysis patients.
2. METHODS
2.2. Participants
The participants of this study were outpatients receiving hemodialysis in the Hemodialysis Unit of E General Hospital in Seoul. Before the research was started, information was provided to all of the patients by posting a notice on the research noticeboard in the hemodialysis unit, and a nurse in the hemodialysis unit recruited patients and delivered a list of applicants to the researcher. The following inclusion criteria were applied: (1) aged between 20 and 75 years, (2) outpatients receiving hemodialysis three times a week for more than 3 months, (3) not being overly sensitive to aroma or aroma oil, (4) exhibiting a gag reflex, and (5) able to understand the purpose of this study and provide written consent to participate in the research voluntarily. The following exclusion criteria were applied: (1) being treated for an intraoral infection or wound, (2) inability to smell due to olfactory dysfunction, (3) inability to gargle with a solution, (4) needing surgery or treatment for medical or surgical complications, (5) unable to breathe through the nose, and (6) participating in another aroma-related study.
In order to prevent selection biases, a researcher without the knowledge about the participants and not involved in participant recruitment or data collection assigned the patients receiving hemodialysis randomly through coin flipping. Gargling treatment was applied in the experimental group by another researcher. Two research assistants collected data by measuring the variables before and after the treatment. The research assistants were blinded to the grouping. The researcher trained these research assistants in how to measure outcome variables until reaching a good inter-rater reliability (Kappa=0.83, p<.001) when measuring these variables on 15 healthy people.
It was estimated that at least 23 participants would be required in each of the experimental and control groups (i.e., at least 46 in total), as calculated using the G* power program (version 3.1.2) for an effect size of .86 which is calculated from a previous study [25], a significance level (α) of 0.05, a test power (1-β) of 0.8, a two-sided test, and a 1:1 assignment to each group. In consideration of a predicted maximum dropout rate of 20%, the present study therefore included 28 participants in each group; all 56 participants completed the research since there were no dropouts.
2.3. Ethical Considerations
This study obtained prior approval from the Institutional Review Board of E General Hospital (approval no. EU 13-46). For ethical protection of the participants, the research assistants explained the purposes and procedure of the research to the participants, promised to observe the guidelines for personal information processing and to maintain confidentiality when collecting and analyzing data and using the results, and requested their voluntary participation. After advising the participants that they could withdraw their participation anytime during the research, their written consent was obtained. After the completion of the last posttest, 20ml of A-Solution was offered to the participants in the control group.
2.4. Measurements
The general, clinical, and halitosis-related characteristics of the participants were surveyed using a questionnaire, xerostomia and subjective halitosis were assessed using a VAS, objective halitosis was measured with a portable halitosis detector, and salivary pH was measured with BTB (bromothymol blue) pH test paper.
2.4.1. Xerostomia
Xerostomia was assessed using a VAS asking about the degree of dryness inside the mouth. A research assistant explained the scale to the participants, which ranged from 0 to 10, and let them place a mark directly on it. The distance in centimeters to the marked point was taken as the measured score, where a high score indicated severe xerostomia.
2.4.2. Salivary pH
Salivary pH was measured with BTB pH test paper (MACHEREY-NAGEL, Düren, Germany: pH 6.0~8.0). This BTB pH test paper has a 0.2 graduation interval, and it can measure pH precisely. The test paper was placed on the participant’s tongue so that it absorbed saliva, and then it was immediately compared with the standardized color chart and the pH value was read based on the closest color.
2.4.3. Objective Halitosis
Objective halitosis was measured using a portable halitosis detector (HC-205, Tanita, Tokyo, Japan). Halitosis is caused mainly by volatile sulfur compounds and hydrocarbon gas contained in hydrogen sulfide, methyl mercaptan, and dimethyl sulfide, and this portable halitosis detector can detect the gases that cause halitosis. The level of halitosis measured by the halitosis detector ranges from level 0 to 5 as follows: level 0, no halitosis; level 1, slight halitosis; level 2, moderate halitosis; level 3, occasional severe halitosis; level 4, severe halitosis; and level 5, very severe halitosis. In measuring halitosis, the participant was instructed to close the mouth for 1 minute to allow volatile sulfur compounds to accumulate. The device was then shaken four or five times to remove odor and moisture inside it, its power button was pressed, and then the participant breathed into the opening for 3 seconds, with this process being repeated twice and the mean of the two measurements calculated.
2.4.4. Subjective Halitosis
Subjective halitosis was the level of halitosis that the participants measured for themselves using a VAS. The scale ranged from “no halitosis at all” (score of 0) to “very severe halitosis” (score of 10). The distance in centimeters to the marked point was taken as the measured score.
2.5. Data Collection Procedure
Data were collected in this study from October 1 to November 15, 2013 according to the procedure described below.
2.5.1. Pretest
A nurse working in the hemodialysis unit of E General Hospital recruited applicants among outpatients receiving hemodialysis and obtained written consent from those who were consistent with the inclusion and exclusion criteria. The research assistants then conducted the pretest using a self-administered questionnaire asking about general, clinical, and halitosis-related characteristics. The participants answered the questionnaire by themselves, but for those who needed assistance due to impaired vision, which is observed commonly in hemodialysis patients, the research assistant read the questions to 5 patients in the experimental (2 patients) and control (3 patients) groups and then marked their answers. In addition, the blood urea level (one of the clinical characteristics used to monitor hemodialysis patients) was obtained from medical records. Xerostomia, subjective halitosis, objective halitosis, and salivary pH were measured by the research assistant using the devices described in section 2.5.2.
2.5.2. Intervention Protocol
The type of aroma to be used in the experiment was selected based on previous studies. The dilution ratio and preparation of A-Solution were determined based on advice obtained from an expert who was a nursing professor and aromatherapist. Considering that the participants were hemodialysis patients, and reflecting the opinion of healthy people and one hemodialysis patient showing that the aroma fragrance and cooling sensation were too strong when the mixture of peppermint, lemon, and tea tree was prepared at a ratio of 2:2:1 and diluted to 0.15% in the pilot test, peppermint, lemon, and tea tree were blended at a ratio of 1:2:1 and the mixture was dissolved in the solvent and diluted to 0.1% with distilled water. The researcher filled a disposable gargling cup with 20 ml of A-Solution and gave it to the experimental group one hour after dialysis. The experimental group gargled with 20 ml of A-Solution for about 15 seconds. They put the 20-ml A-Solution in their mouths and swished it around their mouths by moving their cheeks in and out, and their tongues back and forth to swish the A-Solution back and forth in their mouths. Thereafter, the researcher instructed them to tilt their heads back and, without swallowing the A-Solution, to try to open their mouths and make the “ahhh” sound for 2 or 3 seconds. Then, the researcher instructed them to spit out the A-Solution into another disposable cup. No treatment was applied to the control group. In order to control exogenous variables affecting the dependent variables, both groups skipped tooth brushing after breakfast if they received dialysis in the morning, or after lunch, if they received dialysis in the afternoon. In addition, the participants did not eat food known to induce halitosis (e.g., onion, cheese, meat, and fish) for breakfast for morning-dialysis patients, or for lunch for afternoon-dialysis patients. All of the participants skipped tooth brushing once before receiving dialysis, and they fasted during the test.
2.5.3. Posttests
Xerostomia, salivary pH, and subjective and objective halitosis were measured at 5, 30, 60, and 120 minutes after the experimental treatment in the experimental group, and after the pretest in the control group. Based on previous studies [15, 16, 20], the effect of experimental treatment was measured at 5, 30, and 60 minutes after the treatment, and its long-term effect was measured after 120 minutes.
2.6. Data Analyses
Collected data were analyzed using SPSS (version 23.0 for Windows). The general, halitosis-related, and clinical characteristics of the participants were analyzed using descriptive statistics, and the homogeneity of the two groups was tested using the χ2-test, Fisher’s exact test, and independent t-test for the general, clinical, and halitosis-related characteristics of the participants, and for their xerostomia, subjective halitosis, objective halitosis, and salivary pH. Differences in xerostomia, subjective halitosis, objective halitosis, and salivary pH after the experimental treatment between the experimental and control groups were analyzed using two-way repeated-measures ANOVA.
3. RESULTS
3.1. Homogeneity Tests of the Characteristics and Dependent Variables in the Experimental and Control Groups
The homogeneity tests of the general, halitosis-related, and clinical characteristics of the participants revealed no statistically significant differences between the two groups, indicating that these groups were homogeneous (Table 1). The same results were obtained in the homogeneity tests of their xerostomia, halitosis, and salivary pH (Table 2).
3.2. Hypothesis Testing
3.2.1. Hypothesis 1: Xerostomia will Differ Between the Experimental Group, in which Gargling with A-Solution will be Applied, and the Control Group
The mean score for xerostomia did not differ significantly between the experimental group in which gargling with A-Solution was applied and the control group. The degree of xerostomia in the experimental group decreased after the treatment relative to that in the pretest, while that in the control group was more than twofold higher after 120 minutes than that in the pretest. This resulted in the degree of xerostomia after 120 minutes differing significantly between the two groups (t=–3.01, p=.004), and also between time points 5, 30, 60, and 120 minutes after the treatment (F=9.19, p<.001). The xerostomia score in the experimental group was 2.68±1.22 (mean± SD) in the pretest and had decreased at 5, 30, 60, and 120 minutes after the treatment, while in the control group it was 1.75±2.46 in the pretest and increased gradually to reach 3.96±3.32 after 120 minutes. As a result, xerostomia differed significantly in the interaction between group and time point (F=12.75, p<.001). Therefore, Hypothesis 1―that xerostomia will differ between the experimental and control groups―was supported (Table 3).
3.2.2. Hypothesis 2: Salivary pH will Differ Between the Two Groups
The mean salivary pH differed significantly between the two groups (F=6.64, p=.013). The repeated-measures ANOVA according to time point revealed that salivary pH differed significantly between the pretest and at 5 minutes after the treatment, and also among at 5, 30, 60, and 120 minutes after the treatment (F=5.35, p<.001). The univariate analysis of differences between the two groups at each time point revealed significant differences between them at 30 minutes (t=5.91, p=.018) and 120 minutes (t=6.14, p=.016). The salivary pH in the experimental group was 6.91±0.45 in the pretest and 7.18±0.55 at 5 minutes after the treatment, and remained higher than that in the pretest until 120 minutes after the treatment. The salivary pH in the control group was 7.14±0.46 in the pretest and decreased gradually to 6.88±0.46 at 120 minutes. As a result, salivary pH differed significantly in the interaction between group and time point (F=3.19, p=.014). Therefore, Hypothesis 2-that salivary pH will differ between the experimental and control groups-was supported (Table 3).
| Characteristics | Experimental group (n=28) | Control group (n=28) | 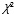 |
p | ||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Mean±SD (Min~Max) |
N (%) | Mean±SD (Min~Max) |
|||||
| General characteristics | Gender | Male | 17(30.4) | - | 17(30.4) | - | - | 1.000 |
| - | Female | 11(19.6) | - | 11(19.6) | - | |||
| Age | 40~49 | 2(3.6) | 61.96±8.84 (31~73) |
6(10.7) | 56.82±12.24 (34~74) |
3.38 | .364a | |
| - | 50~59 | 7(12.5) | 9(16.1) | |||||
| - | 60~69 | 14(25.0) | 9(16.1) | |||||
| - | 70~75 | 5(8.9) | 4(7.1) | |||||
| Education | Elementry school | 6(10.6) | - | 5(8.9) | - | 2.56 | .470 | |
| - | Middle school | 10(17.9) | - | 6(10.6) | - | |||
| - | High school | 6(10.6) | - | 11(19.6) | - | |||
| - | University | 6(10.6) | - | 6(10.6) | - | |||
| Clinical characteristics | Drugb | Antihyper-tensives | 28(50.0) | - | 25(45.0) | - | 1.18 | .395a |
| - | Psychotics | 4(7.2) | - | 6(10.8) | - | |||
| - | Antihistamine | 6(10.8) | - | 10(18.0) | - | |||
| - | Diuretics | 10(18.0) | - | 7(12.6) | - | |||
| Diseaseb | Hypertension | 23(41.4) | - | 25(45.0) | - | 1.77 | 1.000a | |
| - | DM | 22(39.6) | - | 24(43.2) | - | |||
| - | Others | 4(7.2) | - | 5(9.0) | - | |||
| HD duration (year) |
Below 5 | 22(39.3) | - | 21(37.5) | - | 1.00 | 1.000a | |
| - | Over 5 | 6(10.7) | - | 7(12.5) | - | |||
| Subjective health | - | - | 3.00±0.39 (1~4) |
- | 2.79±0.63 (1~4) |
1.54 | .130 | |
| Characteristics related to halitosis | Dental caries |
Yes | 10(17.9) | - | 12(21.4) | - | 0.30 | .785 |
| - | No | 18(32.1) | - | 16(28.6) | - | |||
| Teeth brushing frequency (times/day) |
Below 3 | 18(32.1) | - | 19(33.9) | - | 0.08 | 1.000 | |
| Over 3 | 10(17.9) | - | 9(16.1) | - | ||||
| Drinking | Yes | 0(0.0) | - | 1(1.8) | - | 1.87 | 1.000a | |
| - | No | 28(50.0) | - | 27(48.2) | - | |||
| Smoking | Yes | 1(1.8) | - | 3(5.0) | - | 1.89 | .611a | |
| - | No | 27(48.2) | - | 25(44.6) | - | |||
| Rhinitis | Yes | 5(8.9) | - | 6(10.7) | - | 0.21 | .648 | |
| - | No | 23(41.1) | - | 22(39.3) | - | |||
| BUN level | - | - | 53.71±13.43 (20.5~82.9) |
- | 52.61±16.88 (10.0~81.6) |
0.27 | .788 | |
| Variables | Experimental group (n=28) | Control group (n=28) | t | p |
|---|---|---|---|---|
| Mean±SD | Mean±SD | |||
| Xerostomia | 2.68±1.22 | 1.75±2.46 | 1.79 | .079 |
| Salivary pH | 6.91±0.45 | 7.14±0.46 | -1.82 | .074 |
| Halitosis | - | - | - | - |
| Subjective | 2.61±1.83 | 2.11±1.01 | 1.00 | .322 |
| Objective | 3.00±0.67 | 2.89±0.69 | 0.59 | .556 |
| Variables | Time | Experimental group (n=28) | Control group (n=28) | t | p | Comparison | F | p |
|---|---|---|---|---|---|---|---|---|
| Mean±SD | Mean±SD | |||||||
| Xerostomia | pretest | 2.68±1.22 | 1.75±2.46 | 1.79 | .079 | Group | 1.25 | 0.296 |
| post testI | 1.54±1.26 | 1.86±2.59 | -0.59 | .558 | Time | 9.19 | <.001 | |
| post testII | 2.04±1.60 | 2.46±3.00 | -0.67 | .507 | Group*Time | 12.75 | <.001 | |
| post testIII | 2.07±1.56 | 3.29±3.35 | -1.74 | .088 | - | - | - | |
| post testIV | 1.89±1.50 | 3.96±3.32 | -3.01 | .004 | - | - | - | |
| Salivary pH | pretest | 6.91±0.45 | 7.14±0.46 | -1.82 | .074 | Group | 6.64 | 0.013 |
| post testI | 7.18±0.55 | 7.19±0.55 | 1.87 | .178 | Time | 5.35 | <0.001 | |
| post testII | 7.06±0.49 | 7.00±0.55 | 5.91 | .018 | Group*Time | 3.19 | 0.014 | |
| post testIII | 6.97±0.52 | 6.91±0.57 | 3.13 | .083 | - | - | - | |
| post testIV | 7.05±0.05 | 6.88±0.46 | 6.14 | .016 | - | - | - | |
| Subjective Halitosis | pretest | 2.61±1.83 | 2.11±1.01 | 1.00 | .322 | Group | 18.31 | <0.001 |
| post testI | 0.79±1.17 | 2.29±2.09 | -3.32 | .002 | Time | 3.94 | <0.001 | |
| post testII | 0.79±1.13 | 2.82±2.11 | -4.50 | <.001 | Group*Time | 11.54 | 0.036 | |
| post testIII | 0.93±1.25 | 3.00±2.37 | -4.09 | <.001 | - | - | - | |
| post testIV | 1.00±1.49 | 3.75±2.43 | -5.11 | <.001 | - | - | - | |
| Objective Halitosis | pretest | 3.00±0.67 | 2.89±0.69 | 0.59 | .556 | Group | 27.12 | <.001 |
| post testI | 1.68±0.82 | 2.79±0.69 | -5.48 | <.001 | Time | 27.37 | <.001 | |
| post testII | 1.61±0.88 | 2.64±0.62 | -5.11 | <.001 | Group*Time | 21.62 | <.001 | |
| post testIII | 1.71±0.81 | 2.75±0.65 | -5.30 | <.001 | - | - | - | |
| post testIV | 1.75±0.89 | 3.18±0.95 | -5.83 | <.001 | - | - | - |
3.2.3. Hypothesis 3: Halitosis will Differ Between the Two Groups
3.2.3.1. Subhypothesis 3-1 Subjective Halitosis will Differ Between the Two Groups
The mean level of subjective halitosis differed significantly between the groups (F=18.31, p<.001). According to time points, subjective halitosis differed significantly between the pretest and after 5 minutes, and also among the time points of 5, 30, 60, and 120 minutes after the treatment (F=3.94, p<.001). As for the interaction between group and time point, the level of subjective halitosis in the experimental group was 2.61±1.83 in the pretest, and decreased to 0.79±1.17 at 5 minutes after the treatment and decreased further until 120 minutes, while in the control group it was 2.11±1.01 in the pretest and increased gradually to 3.75±2.43 at 120 minutes after the pretest. The level of subjective halitosis in the experimental group decreased by 1.51 between the pretest and at 120 minutes after the treatment, while in the control group it increased by 1.64 between these two time points, showing that subjective halitosis differed significantly in the interaction between group and time point (F=11.54, p=.036). Therefore, Subhypothesis 3-1-that subjective halitosis will differ between the experimental and control groups-was supported (Table 3).
3.2.3.2. Subhypothesis 3-2: Objective Halitosis will Differ Between the Two Groups
The mean level of objective halitosis differed significantly between the groups (F=27.12, p<.001). According to time points, objective halitosis differed significantly among the pretest and at 30, 60, and 120 minutes after the treatment, and between 120 minutes after the treatment and the pretest and at 30 and 60 minutes after the treatment (F=27.37, p<.001). The level of objective halitosis in the experimental group was 3.00±0.67 in the pretest and 1.68±0.82 at 5 minutes after the treatment, and decreased until 120 minutes after the treatment, while in the control group it was 2.89±0.69 in the pretest and increased gradually to 3.18±0.95 at 120 minutes, showing a significant difference in the interaction between group and time point (F=21.62, p<.001). Therefore, Subhypothesis 32-that objective halitosis will differ between the experimental and control groups-was supported (Table 3).
4. DISCUSSION
This study tested whether gargling with A-Solution reduces xerostomia, subjective halitosis, and objective halitosis, and maintains salivary pH at a normal level in hemodialysis patients, thereby providing the basis for the clinical application of aroma gargling.
When gargling with A-Solution was applied to hemodialysis patients in this study, the degree of xerostomia did not differ between the experimental and control groups, but it did differ significantly across the time points and in the interaction between group and time point. Xerostomia decreased in the experimental group after the treatment compared to that in the pretest, while in the control group it was more than twofold higher at 120 minutes after the pretest than that in the pretest. This is similar to a previous report [21] of the application of aroma gargling to nursing students involving a mixture of peppermint, tea tree, and lemon at a ratio of 2:1:2 and diluted to 0.15% decreasing xerostomia at 60 and 120 minutes after the treatment compared to the saline comparison group. This is also similar to another study [26] finding that when the effects of lemon ice and spring-water ice on thirst and the oral condition was examined in nasal-surgery patients, by providing the experimental group with lemon ice and the control group with spring-water ice twice at an interval of 15 minutes and then measuring after 10 minutes, the level of thirst decreased significantly in both groups but the decrease was significantly larger in the lemon-ice group. In the present study, the participants gargled once only with the mixture of peppermint, lemon, and tea tree at a ratio of 1:2:1 diluted to 0.1%, but xerostomia was still reduced markedly at 5 minutes after gargling. Xerostomia then subsequently increased gradually at 30 and 60 minutes after gargling, and decreased at 120 minutes. Xerostomia in the control group increased over times, result in a significant difference between the two groups after 120 minutes, demonstrating that A-Solution is effective for up to 120 minutes. It is believed that the sour taste of lemon, which was commonly included in the interventions applied in the previous study [20, 21] and this study, increased salivary secretion. The finding of the previous study [20] that xerostomia decreased at 20 minutes after two treatments demonstrates the short-term effect of aroma. In general, xerostomia increases during the hemodialysis because fluid removal in the body increases [27], but the present study has demonstrated the long-term effect of gargling for up to 120 minutes by providing gargling treatment at a mixing ratio lower than that used in the previous study [20]. Since this study measured subjective dryness and thirst using a visual analog scale, as in the previous studies [20, 21, 26] involving nursing students and involving preoperative NPO patients and nasal-surgery patients using lemon ice, further research may be necessary that measures xerostomia using objective physiological indicators such as the salivary flow rate.
When salivary pH was measured after gargling with A-Solution, significant differences were observed between the experimental and control groups, across time points, and in the interaction between group and time point. Repeated-measures ANOVA revealed a significant difference across time points. Univariate analysis of each time point using the pretest salivary pH as a covariate found that the salivary pH values at 30 and 60 minutes after the treatment were significantly lower in the control group. This is consistent with the results [20] obtained by applying aroma gargling (30 ml) twice to preoperative NPO (“nil per os” [nothing by mouth]) patients using a mixture of peppermint, lemon, and tea tree at a ratio of 1:2:2 and diluted to 0.125%, in which their salivary pH after 20 minutes was decreased about three times more in the control group than in the experimental group. A further study [22] provided oral care to community-dwelling elderly four times a day for 2 weeks using 20 ml of aroma prepared using a mixture of tea tree, mandarin, and myrrh at a ratio of 5:4:1 and diluted to 0.5%, and found that the salivary pH was increased in the experimental group just after the treatment. The pretest salivary pH was 6.72 in the experimental group and 6.63 in the control group in the previous study [20] involving preoperative NPO patients, and 6.91 in the experimental group in the present study. These results did not support the hypothesis that the saliva of hemodialysis patients is more acidic in general. However, in the study involving surgical patients, the salivary pH decreased in both the experimental group and the control group, but the decrease was smaller in the experimental group, and in the study [22] involving community-dwelling elderly, the salivary pH increased in the experimental groups after the treatment but decreased in the control groups, as in the present study; all of these findings suggest that aroma gargling is a useful intervention for increasing the salivary pH. This study measured the salivary pH using BTB pH test paper, the previous studies [20, 21] involving nursing students and surgical patients used BTB and BCP (bromocresol purple) pH test paper, and the previous study [26] involving community-dwelling elderly using frozen saliva and a pH meter in the laboratory. The results of the present study that salivary pH increased after the treatment in the experimental group and decreased in the control group are consistent with the findings of previous studies [28] that halitosis is generally associated with a lower salivary flow and the acidification of saliva. Thus, it is considered necessary to generalize these results through replication studies and to perform additional research on the associations among halitosis, xerostomia, and salivary pH.
The level of subjective halitosis measured after gargling with A-Solution differed significantly between the two groups, across time points, and in the interaction between group and time point. The level of subjective halitosis decreased by 1.29 in the experimental group at 120 minutes after the treatment compared to that in the pretest, and increased by 1.64 in the control group at 120 minutes after the pretest, showing that gargling with A-Solution lowered the level of subjective halitosis. Previous studies [20, 21, 25, 28] have used the following aroma mixtures and dilution ratios: (1) peppermint, lemon, and tea tree diluted to 0.125% in preoperative NPO patients; (2) geranium, lavender, tea tree, and peppermint diluted to 0.5% in hospice patients; (3) peppermint, lemon, and tea tree diluted to 0.15% in nursing students; and (4) peppermint, lemon, and tea tree diluted to 0.125% in intensive care (ICU) patients; the corresponding numbers of treatments and measurement time points were (1) at 5 and 60 minutes after one oral-care treatment, (2) at 20 minutes after two gargling treatments, (3) at 60 and 120 minutes after three gargling treatments, and (4) for 7 days of oral care using gauze twice daily, respectively. The tools used in these previous studies were VASs, as in the present study. The previous study [25] involving ICU patients found that subjective halitosis was significantly less severe in the aroma group than in the control group that used Tantum. In the study [20] involving preoperative NPO patients, both the experimental and control groups showed an increase in the level of subjective halitosis, but this increase was 50% in the experimental group and 300% in the control group. These results suggest that aroma gargling is an intervention that both mitigates halitosis and inhibits the aggravation of halitosis, and the lowered level of subjective halitosis observed in several previous studies is believed to be attributable to the effect of aroma gargling against halitosis.
The level of objective halitosis decreased from 3.0 in the pretest to 1.75 at 120 minutes after the treatment in the experimental group in the present study, and increased by 0.29 in the control group, with this between the two groups being statistically significant. The level of objective halitosis also differed significantly across time points; that is, it decreased until 30 minutes after the treatment, and then increased gradually at 60 and 120 minutes, but remained lower than that in the pretest. This result is consistent with a previous report [20] that the level of halitosis and xerostomia differed significantly between the groups until 20 minutes after applying aroma gargling to preoperative NPO patients. With regard to research tools, those studies [20, 21, 28] involving preoperative NPO patients, nursing students, and community-dwelling elderly used a portable halitosis detector, as in the present study. The study involving preoperative NPO patients compared between measurements made with a portable halitosis detector and a VAS, and found consistency between the results obtained using these two techniques. In addition, the pretest level of objective halitosis was 2.53 in the study involving nurses, 2.09 in that involving preoperative NPO patients, and 3.00 in the present study involving hemodialysis patients, suggesting that the level of halitosis is higher in hemodialysis patients than in other groups and therefore that these patients require their own interventions for halitosis. In the present study, the aroma oil was diluted thinly to 0.1% based on consideration of the lowered renal excretion capacity of hemodialysis patients, but this solution was still effective in reducing halitosis. In general, the level of halitosis may decrease concomitantly with the decreasing blood urea level as dialysis proceeds, but because the blood urea level before dialysis did not differ significantly between the groups, the lower level of objective halitosis in the experimental group is believed to be attributable to the effect of gargling with A-Solution. Moreover, the level of halitosis decreased for up to 120 minutes after a single treatment in the experimental group, while it increased over time in the control group, which further suggests that aroma gargling has a long-lasting effect. In order to confirm the effect of aroma gargling for longer than 1 hour as tested in a previous study [20, 21], the present study measured the effect until 120 minutes after the treatment and observed a significant difference between the experimental and control groups from 5 until 120 minutes after the treatment. Thus, this intervention is believed to have an effect that is both rapid and durable, and is safe to apply to hemodialysis patients without inducing nephrotoxicity. In summary, gargling with A-Solution as applied in this study significantly reduced both subjective and objective halitosis between the groups, across time points, and in the interaction between group and time point. A previous study found that an aroma necklace containing lavender and bergamot oil eased the subjective stress and state anxiety of hemodialysis patients, but did not affect their physiological responses, which implies that the effect of an intervention may be influenced by the pathological condition or lifestyle of the patients. Therefore, future research needs to be designed so as to control exogenous variables that may affect the results. As presented above, gargling with A-Solution applied to hemodialysis patients reduced their xerostomia and halitosis and increased the salivary pH over time in the experimental group, with the opposite effects being observed in the control group. Several previous studies have demonstrated the effects of applying aroma gargling to different groups of participants, but the present study is the first to have applied an aromatherapy for the oral care of hemodialysis patients with severe xerostomia and halitosis, and hence the present findings are considered highly meaningful. If a replication study is conducted for comparing the correlation between xerostomia and halitosis and salivary pH as mentioned above, aroma gargling may be used as a clinical nursing intervention to address oral problems in hemodialysis patients.
CONCLUSION
This study examined the effects of gargling with A-Solution on xerostomia, halitosis, and salivary pH in patients receiving hemodialysis for chronic renal failure. The obtained results indicate that gargling with A-Solution reduced subjective and objective halitosis, partially eased xerostomia, and increased salivary pH for up to 120 minutes after the treatment in hemodialysis patients. Thus, gargling with A-Solution is considered an effective nursing intervention for reducing subjective and objective halitosis and mitigating xerostomia in patients receiving hemodialysis.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study obtained prior approval from the Institutional Review Board of E General Hospital (approval no. EU 13-46).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all the participants prior to publication.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENT
We sincerely thank the nurses and patients in the Hemodialysis Unit of E General Hospital and those who participated in this study.


